
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Iwona Sidorkiewicz | + 1602 word(s) | 1602 | 2020-09-16 12:47:21 | | | |
| 2 | Peter Tang | Meta information modification | 1602 | 2020-09-18 06:13:41 | | |
Video Upload Options
Endometrial cancer (EC) remains one of the most common cancers of the female reproductive system. Epidemiological and clinical data implicate insulin resistance (IR) and its accompanying hyperinsulinemia as key factors in the development of EC. MicroRNAs (miRNAs) are short molecules of non-coding endogenous RNA that function as post-transcriptional regulators. Accumulating evidence has shown that the miRNA expression pattern is also likely to be associated with EC risk factors.
1. Introduction
Endometrial cancer (EC) is the most common gynecological cancer in developed countries, with annual rates continuing to increase. It is estimated that more than 60,000 new EC diagnoses and 11,000 deaths from the disease occur in the United States alone every year [1]. However, the etiology of this disease is still not fully understood [1][2]. EC has been generally divided into two clinical categories. The first is classified as type I, which represents the vast majority (80–90%) of cases and is associated with a hyperplastic, low-grade, estrogen-related endometrium. It occurs primarily in obese pre-, peri-, and early postmenopausal women, and is associated with a good prognosis. Type II is characterized by a non-estrogenic, high-grade atrophic endometrium, which is also less well-differentiated. It occurs mostly in postmenopausal women and has a high risk of relapse and metastatic disease [3]. There are several histologic types of EC, and the most common endometrioid carcinoma tends to have a favorable prognosis. Other histotypes (such as serous or clear cell carcinoma) of EC are associated with a poor prognosis [4][5]. It was initially noted that type I EC generally presents an endometrioid morphology, whereas type II cancers are characterized by non-endometrioid histology, predominantly serous. However, this classical distinction into two EC types has been challenged by long-term follow-up of patients with cancer of endometrioid histology and grades 2 and 3 of differentiation, whose survival turned out to be worse than expected [6][7]. In line with this, Setiawan et al. observed that the risk factor patterns of high-grade endometrioid tumors and type II tumors were similar [3]. Currently, only endometrioid grade 1 (well differentiated) EC is considered to be type I, with the remainder of EC cases being included into type II.
Although EC is generally considered to be hormone-sensitive, its development is widely considered to also be regulated by environmental and lifestyle factors. One of this cancer’s risk factors is insulin resistance (IR), a prominent component in many metabolic disorders, including prediabetes, type 2 diabetes mellitus (T2DM), metabolic syndrome, and polycystic ovary syndrome (PCOS) [8][9][10][11]. IR is a condition of reduced sensitivity of insulin-responsive tissues to insulin, which leads to an increase in blood insulin and glucose concentrations. According to the International Diabetes Federation Diabetes Atlas, the global prevalence of T2DM developed from IR continues to be on the increase [12]. Hyperinsulinemia can trigger many physiological effects that drive carcinogenesis, as insulin is a major anabolic hormone that can stimulate cell proliferation [8]. Reduced receptor binding and decreased insulin receptor-mediated transduction lead to hyperinsulinemia which, in turn, triggers the deregulation of many metabolic pathways [13]. The exact molecular mechanisms linking IR and EC are still uncertain. However, the direct effect(s) on endometrial cells of insulin and insulin-like growth factors (IGFs), as well as of alterations in the mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) and in the complex of phosphatidylinositol 3-kinase (P13K)–phosphatase and tensin homolog deleted on chromosome 10 (PTEN)–protein kinase B (Akt) signaling pathways, may play crucial roles [9][14][15].
Cancer development is also associated with epigenetic dysregulation, occurring at the earliest stage of cancer [16]. The most common epigenetic modifications are DNA methylation, histone methylation and acetylation, and the actions of non-coding RNAs, including microRNAs (miRNAs). All of them can regulate multiple genes and are involved in various important signaling pathways [17]. miRNAs belong to a class of highly conserved, sequence-specific, single-stranded, endogenous small non-coding RNAs, which bind to the 3’ end of the target mRNAs to induce their destabilization, degradation, and/or translation inhibition [18]. Deregulation of miRNA profiles has been implicated in a variety of cellular processes, including cancer development. Therefore, miRNAs have been drawing attention for their potential usefulness as diagnostic and/or prognostic markers [19][20].
2. Clinical Importance of the Association between Insulin Resistance and Dndometrial Cancer
Generally, IR is a principal pathophysiological process that relates not only to diabetes but also to prediabetes, as well as preclinical hyperinsulinemia and dysglycemia of varied degrees. IR has been defined as the resistance of target organs to the actions of insulin so that increased concentrations of this hormone are necessary to obtain a normal biological effect [21]. Accordingly, IR is the primary cause of T2DM and occurs years before its clinical manifestation [22]. This prediabetic state plays an important role in the development and progression of some types of cancers, including breast, prostate, colorectal, and endometrial neoplasia [23]. There is accumulating evidence that the risk factors for IR are also risk factors for EC, which strongly suggests that the development of IR and EC may be parallelly promoted at the same time. A meta-analysis conducted by Saed et al. demonstrated that diabetes increases the risk of EC by 72% [24]. Another work, a meta-analysis of 16 studies (3 cohort and 13 case-control studies), found that diabetes is associated with a 2.1-fold increase in the relative risk for EC [25]. Notably, a higher prevalence of EC was demonstrated in non-diabetic women with IR [26]. Decreased serum adiponectin (a polypeptide hormone increasing the cell’s insulin sensitivity and a surrogate marker for IR) concentration was found to be independently and inversely correlated with EC occurrence [27][28]. It has also been established that the EC risk increases quite shortly following the diagnosis of IR and diabetes; that is, approximately past 6 months after their detection [29]. Elevated levels of insulin in prediabetic and diabetic patients seem to affect their cancer risk rather quickly [30]. Similarly, epidemiological evidence shows that the presence of accompanying diseases substantially influences EC risk estimations [10]. For instance, the relationship between diabetes and EC incidence can be largely promoted by increased body weight [23]. In their pooled analysis of cohort studies, Stocks et al. found direct linear relationships of body mass index (BMI), blood pressure, blood glucose, triglycerides, and total cholesterol concentrations with EC risk [31].
3. Insulin Resistance as a Driving Force for Endometrial Cancer
Over past decades, hyperinsulinemia and IR have been implicated as playing a major role in diabetes-promoted cancers. Multiple studies were able to demonstrate a direct association between IR and the incidence of EC with several biological mechanisms as a result of their common regulation by molecular factors (such as mediators of inflammation and adipokines) [32][33][34]. Figure 1 presents a model of links between metabolic alterations in the development of this malignancy, highlighting the roles of changes in the insulin and IGF system and mediators of inflammation.
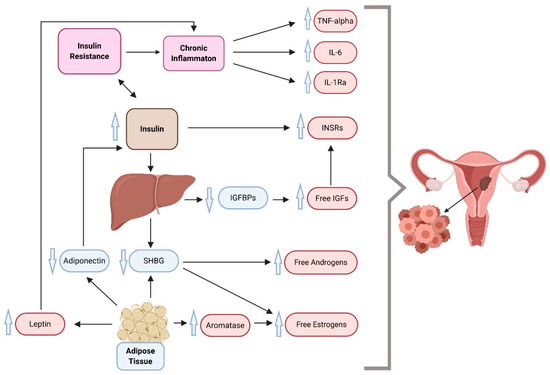
Figure 1. A proposed multidimensional model of endometrial cancer development, which suggests insulin resistance, inflammation, and overweight/obesity as driving forces behind cancer. IGFs, insulin-like growth factors; IGFBPs, insulin-like growth factor binding proteins; IL-1Ra, interleukin 1 receptor antagonist; IL-6, interleukin 6; INSR, insulin receptor; SHBG, sex hormone-binding globulin; TNF-alpha, tumor necrosis factor-alpha. Compiled from References [27][35][36][37][38][39].
4. miRNAs in Both Insulin Resistance and Endometrial Cancer
Post-transcriptional regulation by miRNAs is of interest as a mechanism to silence gene expression [40]. Aberrant expression of the miRNA profile plays a key role in a wide variety of physiological processes, including cell proliferation, apoptosis, and tissue differentiation [41]. Yet, deregulation in miRNA biogenesis and function have been shown to modulate many fundamental signaling pathways, including insulin synthesis, secretion, and signal transduction, and therefore, specific miRNA patterns are likely to play a role in the development of IR and related metabolic complications [42]. Importantly, miRNA-mediated insulin signaling modulation is tissue- and cell-specific, with distinct miRNAs modulating components of the insulin transduction pathway only in some tissues or cells. The basis for IR is multifactorial and includes obesity, inflammation, mitochondrial dysfunction, endoplasmic reticulum stress, oxidative stress, lipotoxicity/hyperlipidemia, genetic background, and hypoxia. These factors contribute quite differently to the disruption of insulin signaling [43].
Various conditions are caused by dysregulation of gene networks due to changes in miRNA expression, and the association between miRNAs and cancer is currently under vivid investigation [44]. miRNAs regulate cell metabolic processes either directly by targeting key molecules of metabolic pathways (transporters and enzymes, including kinases), or indirectly by modulating the expression of important transcription factors [45].
On the one hand, EC molecular subtypes have been shown to demonstrate distinct miRNA signatures. These miRNA signatures are reduced, and particular levels of depletion are characteristic for particular EC subtypes [46]. In summary, many miRNAs, either circulating or of tissue origin, have been found dysregulated in EC.
On the other hand, at least several miRNAs are known to be involved in the pathogenesis of cancer. As for endometrial neoplasia, a 4-miRNA signature (miR-4758, miR-876, miR-142, miR-190b) has been established as an independent prognostic factor for overall survival in EC patients (area under the curve (AUC) of receiver operating characteristic (ROC) curve was 0.7 at 5-year overall survival) [47]. By contrast, based on their systematic review, Donkers et al. proposed miR-205, the whole miR-200 family, miR-135b, miR-182, miR-183, and miR-223 as promising diagnostic biomarkers in EC [48]. Such studies were performed in the hope that the expression pattern of miRNA would become an early diagnostic and prognostic biomarker, whilst particular miRNAs could be identified as novel therapeutic targets.
5. Conclusions
Here we highlight changes in miRNA involved in both IR and EC. In support of the possible role of miRNA in both conditions, our careful literature search found that dysregulation of at least 13 miRNAs has been ascribed to both IR and EC. Therefore, miRNA could represent a potential molecular link between the metabolic alterations related to IR and EC. There is a reasonable possibility for miRNAs to become a predictive factor of future EC in IR patients.
References
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108, doi:10.1016/S0140-6736(15)00130-0.
- Memon, A.; Paudyal, P. Epidemiology of endometrial cancer. Endometrial Cancer Curr. Epidemiol. Detect. Manag. 2014, doi:10.1007/978-1-4471-1389-8_13.
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type i and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618, doi:10.1200/JCO.2012.48.2596.
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Villella, J.; Herzog, T.; et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014, 134, 385–293, doi:10.1016/j.ygyno.2014.05.018.
- McAlpine, J.; Leon-Castillo, A.; Bosse, T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018, 244, 538–549, doi:10.1002/path.5034.
- Voss, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol. Oncol. 2012, 124, 15–20.
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278, doi:10.1016/S1470-2045(13)70591-6.
- Orgel, E.; Mittelman, S.D. The links between insulin resistance, diabetes, and cancer. Curr. Diabetes Rep. 2013, 13, 213–222, doi:10.1007/s11892-012-0356-6.
- Shafiee, M.N.; Khan, G.; Ariffin, R.; Abu, J.; Chapman, C.; Deen, S.; Nunns, D.; Barrett, D.A.; Seedhouse, C.; Atiomo, W. Preventing endometrial cancer risk in polycystic ovarian syndrome (PCOS) women: Could metformin help? Gynecol. Oncol. 2014, 132, 248–253, doi:10.1016/j.ygyno.2013.10.028.
- Kitson, S.J.; Gareth Evans, D.; Crosbie, E.J. Identifying high-risk women for endometrial cancer prevention strategies: Proposal of an endometrial cancer risk prediction model. Cancer Prev. Res. 2017, 10, 1–13, doi:10.1158/1940-6207.CAPR-16-0224.
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757, doi:10.1016/j.ygyno.2012.03.032.
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843, doi:10.1016/j.diabres.2019.107843.
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An early indicator of metabolic dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747, doi:10.1210/js.2019-00065.
- Shafiee, M.N.; Chapman, C.; Barrett, D.; Abu, J.; Atiomo, W. Reviewing the molecular mechanisms which increase endometrial cancer (EC) risk in women with polycystic ovarian syndrome (PCOS): Time for paradigm shift? Gynecol. Oncol. 2013, 131, 489–492, doi:10.1016/j.ygyno.2013.06.032.
- Shafiee, M.N.; Seedhouse, C.; Mongan, N.; Chapman, C.; Deen, S.; Abu, J.; Atiomo, W. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Mol. Cell. Endocrinol. 2016, 424, 94–101, doi:10.1016/j.mce.2016.01.019.
- Banno, K.; Kisu, I.; Yanokura, M.; Masuda, K.; Kobayashi, Y.; Ueki, A.; Tsuji, K.; Yamagami, W.; Nomura, H.; Susumu, N.; et al. Endometrial cancer and hypermethylation: Regulation of DNA and MicroRNA by epigenetics. Biochem. Res. Int. 2012, 2012, 738274, doi:10.1155/2012/738274.
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenet. 2018, 10, 59, doi:10.1186/s13148-018-0492-1.
- Valihrach, L.; Androvic, P.; Kubista, M. Circulating miRNA analysis for cancer diagnostics and therapy. Mol. Asp. Med. 2019, 72, 100825, doi:10.1016/j.mam.2019.10.002.
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469, doi:10.1016/j.molmed.2014.06.005.
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561, doi:10.2174/138920210793175895.
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223, doi:10.1152/physrev.00063.2017.
- Tabak, A.G.; Herder, C.; Rathman, W.; Brunner, E.J.; Kivimaki, M. Prediabetes : A high-risk state for developing diabetes. Lancet 2014, 379, 2279–2290, doi:10.1016/S0140-6736(12)60283-9.Prediabetes.
- Lai, Y.; Sun, C. Association of abnormal glucose metabolism and insulin resistance in patients with atypical and typical endometrial cancer. Oncol. Lett. 2018, 15, 2173–2178, doi:10.3892/ol.2017.7590.
- Saed, L.; Varse, F.; Baradaran, H.R.; Moradi, Y.; Khateri, S.; Friberg, E.; Khazaei, Z.; Gharahjeh, S.; Tehrani, S.; Sioofy-Khojine, A.B.; et al. The effect of diabetes on the risk of endometrial Cancer: An updated a systematic review and meta-analysis. BMC Cancer 2019, 19, 527, doi:10.1186/s12885-019-5748-4.
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 2007, 50, 1365–1374, doi:10.1007/s00125-007-0681-5.
- Sun, W.; Lu, J.; Wu, S.; Bi, Y.; Mu, Y.; Zhao, J.; Liu, C.; Chen, L.; Shi, L.; Li, Q.; et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am. J. Cancer Res. 2016, 6, 2334–2344.
- Soliman, P.T.; Wu, D.; Tortolero-Luna, G.; Schmeler, K.M.; Slomovitz, B.M.; Bray, M.S.; Gershenson, D.M.; Lu, K.H. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer 2006, 106, 1376–2381, doi:10.1002/cncr.21866.
- Zeng, F.; Shi, J.; Long, Y.; Tian, H.; Li, X.; Zhao, A.Z.; Li, R.F.; Chen, T. Adiponectin and Endometrial Cancer: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2015, 36, 1670–1678, doi:10.1159/000430327.
- Lukanova, A.; Zeleniuch-Jacquotte, A.; Lundin, E.; Micheli, A.; Arslan, A.A.; Rinaldi, S.; Muti, P.; Lenner, P.; Koenig, K.L.; Biessy, C.; et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP-1, -2 and -3 and risk of endometrial cancer. Int. J. Cancer 2004, 108, 262–268, doi:10.1002/ijc.11544.
- Burzawa, J.K.; Schmeler, K.M.; Soliman, P.T.; Meyer, L.A.; Bevers, M.W.; Pustilnik, T.L.; Anderson, M.L.; Ramondetta, L.M.; Tortolero-Luna, G.; Urbauer, D.L.; et al. Prospective evaluation of insulin resistance among endometrial cancer patients. Am. J. Obstet. Gynecol. 2011, 204, e1–e7, doi:10.1016/j.ajog.2010.11.033.
- Stocks, T.; Bjørge, T.; Ulmer, H.; Manjer, J.; Häggström, C.; Nagel, G.; Engeland, A.; Johansen, D.; Hallmans, G.; Selmer, R.; et al. Metabolic risk score and cancer risk: Pooled analysis of seven cohorts. Int. J. Epidemiol. 2015, 44, 1353–1363, doi:10.1093/ije/dyv001.
- Nead, K.T.; Sharp, S.J.; Thompson, D.J.; Painter, J.N.; Savage, D.B.; Semple, R.K.; Barker, A.; Perry, J.R.B.; Attia, J.; Dunning, A.M.; et al. Evidence of a causal sssociation between insulinemia and endometrial cancer: A mendelian randomization analysis. J. Natl. Cancer Inst. 2015, 107, djv178, doi:10.1093/jnci/djv178.
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers Basel 2020, 12, 1191, doi:10.3390/cancers12051191.
- Garikapati, K.K.; Ammu, V.V.V.R.K.; Krishnamurthy, P.T.; Chintamaneni, P.K.; Pindiprolu, S.K.S.S. Type-II endometrial cancer: Role of adipokines. Arch. Gynecol. Obstet. 2019, 300, 239–249, doi:10.1007/s00404-019-05181-1.
- Kaya, S.; Kaya, B.; Keskin, H.L.; Kayhan Tetik, B.; Yavuz, F.A. Is there any relationship between benign endometrial pathologies and metabolic status? J. Obstet. Gynaecol. Lahore 2019, 39, 176–183, doi:10.1080/01443615.2018.1469606.
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A.; et al. Obesity, inflammatory markers, and endometrial cancer risk: A prospective case—Control study. Endocr. Relat. Cancer 2010, 17, 1007–1019, doi:10.1677/ERC-10-0053.
- Dossus, L.; Lukanova, A.; Rinaldi, S.; Allen, N.; Cust, A.E.; Becker, S.; Tjonneland, A.; Hansen, L.; Overvad, K.; Chabbert-Buffet, N.; et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—A factor analysis. Am. J. Epidemiol. 2013, 177, 787–799, doi:10.1093/aje/kws309.
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as link factor between adipose tissue and cancer. Int. J. Mol. Sci. 2019, 20, 839, doi:10.3390/ijms20040839.
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591, doi:10.1038/nrc1408.
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712, doi:10.3390/ijms17101712.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. Lausanne 2018, 9, 402, doi:10.3389/fendo.2018.00402.
- Nigi, L.; Grieco, G.E.; Ventriglia, G.; Brusco, N.; Mancarella, F.; Formichi, C.; Dotta, F.; Sebastiani, G. MicroRNAs as regulators of insulin signaling: Research updates and potential therapeutic perspectives in type 2 diabetes. Int. J. Mol. Sci. 2018, 19, 3705, doi:10.3390/ijms19123705.
- Khan, S.; Wang, C.H. ER stress in adipocytes and insulin resistance: Mechanisms and significance (Review). Mol. Med. Rep. 2014, 10, 2234–2240, doi:10.3892/mmr.2014.2532.
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30, doi:10.1002/jcp.25056.
- La Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purrello, M.; Di Pietro, C. Non-coding RNAs in endometrial physiopathology. Int. J. Mol. Sci. 2018, 19, 2120, doi:10.3390/ijms19072120.
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A microRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207, doi:10.1016/j.cell.2010.05.017.
- Wu, Y.S.; Lin, H.; Chen, D.; Yi, Z.; Zeng, B.; Jiang, Y.; Ren, G. A four-miRNA signature as a novel biomarker for predicting survival in endometrial cancer. Gene 2019, 697, 86–93, doi:10.1016/j.gene.2019.01.046.
- Donkers, H.; Bekkers, R.; Galaal, K. Diagnostic value of microRNA panel in endometrial cancer: A systematic review. Oncotarget 2020, 11, 2010–2023, doi:10.18632/oncotarget.27601.





