Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuuki Hata | + 2311 word(s) | 2311 | 2022-03-01 03:09:24 | | | |
| 2 | Conner Chen | -13 word(s) | 2298 | 2022-03-16 01:35:04 | | | | |
| 3 | Conner Chen | -22 word(s) | 2289 | 2022-03-16 01:35:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hata, Y. Heparin–Protamine Particles for Biomedical Application. Encyclopedia. Available online: https://encyclopedia.pub/entry/20581 (accessed on 08 February 2026).
Hata Y. Heparin–Protamine Particles for Biomedical Application. Encyclopedia. Available at: https://encyclopedia.pub/entry/20581. Accessed February 08, 2026.
Hata, Yuuki. "Heparin–Protamine Particles for Biomedical Application" Encyclopedia, https://encyclopedia.pub/entry/20581 (accessed February 08, 2026).
Hata, Y. (2022, March 15). Heparin–Protamine Particles for Biomedical Application. In Encyclopedia. https://encyclopedia.pub/entry/20581
Hata, Yuuki. "Heparin–Protamine Particles for Biomedical Application." Encyclopedia. Web. 15 March, 2022.
Copy Citation
Heparin and protamine are a clinically relevant pair of biomolecules. Heparin is a mixture of linear anionic polysaccharides with many sulfate groups. In cardiac and vascular surgery, the use of the anticoagulant is followed by the administration of protamine, a small arginine-rich cationic protein, to neutralize the heparin. The fact that pharmaceutical grade heparin and protamine are commercially available and clinically used in cardiac and vascular surgery makes these biomolecules attractive as building blocks for in vivo applications.
heparin
protamine
molecular self-assembly
nanoparticle
microparticle
polyelectrolyte complex
biomedical application
1. Introduction
Biomolecules, such as proteins, nucleic acids, lipids and carbohydrates, form sophisticated assemblies via intermolecular interactions and constitute living organisms in nature. The self-assembly ability, structural diversity, and excellent functionality of biomolecules make them attractive as building blocks for creating artificial materials [1][2][3][4][5][6][7][8][9][10]. In fact, assembled biomolecular materials have found practical applications; clinical examples include collagen sponges used for hemostasis and wound healing [11][12] and lipid nanoparticles constituting drug products (e.g., COVID-19 vaccines) [13][14][15][16]. In addition to these clinically used materials composed of a single class of biomolecules, multicomponent assemblies have been explored to access new material properties and morphologies. For instance, the combination of proteins and nucleic acids is of interest in nanotechnology [17][18], while composites of lipid nanoparticles and polysaccharide hydrogels have been investigated as drug carriers [19]. These studies demonstrate that multicomponent biomolecular systems offer opportunities to generate a broad spectrum of functional materials.
Heparin and protamine are a clinically relevant pair of biomolecules. Heparin is a mixture of linear anionic polysaccharides with many sulfate groups and has been used clinically as an anticoagulant for more than 70 years [20][21][22][23]. In cardiac and vascular surgery, the use of the anticoagulant is followed by the administration of protamine, a small arginine-rich cationic protein, to neutralize the heparin [23][24][25][26]. The fact that pharmaceutical grade heparin and protamine are commercially available and clinically used in cardiac and vascular surgery makes these biomolecules attractive as building blocks for in vivo applications. The neutralization effect is a consequence of complex formation between cationic protamine and anionic heparin via electrostatic interactions and other intermolecular interactions. The complex formation in blood indicates that heparin and protamine can coassemble robustly even in crowded environments. Furthermore, the fact that the complexes form in patients’ blood suggests that heparin–protamine coassemblies have good biocompatibility.
2. Drug Carriers
Heparin–protamine particles are useful as carriers for proteins due to their ability to adsorb proteins via electrostatic interactions and other intermolecular interactions. The particles can preserve loaded proteins from degradation by protease and from heat inactivation [27]. Moreover, loaded proteins are released from the particles in a controlled manner, probably through enzymatic degradation of heparin and protamine.
2.1. Intact (Nonmodified) Particles
The potential of heparin–protamine particles as drug carriers has been investigated, especially for fibroblast growth factor (FGF)-2, a protein that stimulates cell proliferation and is used clinically in wound care [28][29][30]. FGF-2 has the advantageous ability to strongly bind to heparin and heparin-like molecules and, moreover, is activated by its binding to heparin [20][21][31][32]. These characteristics have prompted people to use heparin–protamine particles as carriers of FGF-2 for various applications.
FGF-2-containing heparin–protamine nanoparticles were used for the treatment of crush syndrome [33]. A rat model of crush syndrome was prepared by compressing the hind limbs of anesthetized rats using a device, followed by the local administration of FGF-2-containing heparin–protamine nanoparticles. The treated rats exhibited a higher score in motor function, better blood flow, a higher number of blood vessels, and faster recovery of muscle tissue than rats administered FGF-2 alone (i.e., without heparin and protamine). Another study investigated the potential of heparin–protamine carriers for wound care associated with radiation therapy [34]; although radiation therapy is effective for cancer treatment, radiation exposure tends to cause a delay in wound healing as a side effect. Cutaneous full-thickness defect wounds in the backs of rats were made with a punch and a sharp blade. Although X-ray irradiation delayed wound healing, FGF-2-containing heparin–protamine nanoparticle administration prior to irradiation led to a significantly shorter delay accompanied by vascularization, fibrous tissue formation, and fewer apoptotic dermal fibroblasts. Studies on crush syndrome and irradiated wounds demonstrated that FGF-2-containing heparin–protamine particles can promote the healing of various kinds of injury.
FGF-2-containing heparin–protamine nanoparticles were shown to promote hair growth in a clinical study (Figure 1) [35]. Twelve participants with thin hair transdermally applied FGF-2-containing nanoparticle dispersions to the skin of their scalps twice a day for 6 months, resulting in an increase in the mean diameter of their hairs. Objective improvements in thin hair were observed in two cases. Additionally, nine participants experienced greater bounce and hair resilience. Thus, the transdermal application of FGF-2-containing heparin–protamine nanoparticles to the scalp has potential as a new treatment for alopecia.
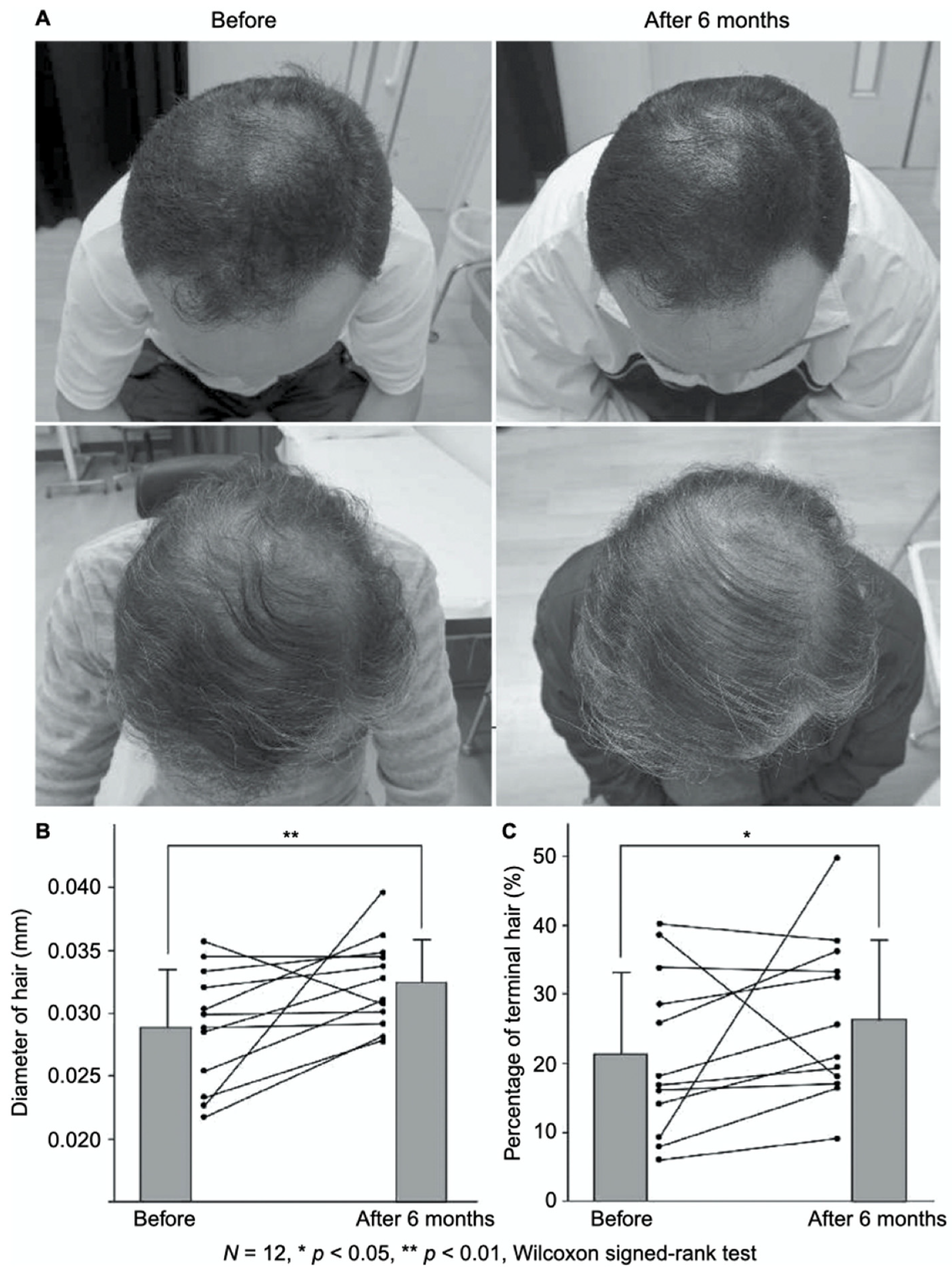
Figure 1. Promotion of hair growth by FGF-2-containing heparin–protamine nanoparticles. (A) Representative photographs of improved cases after 6 months of treatment. Increases in (B) the hair diameter and (C) the percentage of terminal hair.
Heparin–protamine particles can carry not only FGF-2 but also other proteins. In fact, various growth factors contained in platelet-rich plasma were loaded into heparin–protamine particles [36]. Notably, many growth factors in platelet-rich plasma exhibit heparin-binding ability and are activated upon binding, similar to FGF-2. The resultant complexes containing growth factors from platelet-rich plasma were administered to split-thickness skin graft donor site wounds in male rats [37]. The treatment effectively promoted epithelialization and new vessel formation, suggesting that platelet-rich plasma-containing heparin–protamine particles are useful in healing split-thickness skin wounds.
2.2. Nonchemically Modified Particles
The nonchemical modification of heparin–protamine particles has been demonstrated to be a promising strategy for creating advanced drug carriers. Nonchemical modification strategies are generally advantageous in terms of simplicity compared with the chemical modification strategies described in the next subsection. Additionally, the use of US Food and Drug Administration (FDA)-approved and clinically used drugs (i.e., intact heparin and protamine) may contribute to shortening the time required for safety evaluation, even for off-label use.
Heparin–protamine complexes tend to have a net negative charge because anionic heparin is the major component; a high fraction of protamine causes precipitation rather than particle formation. The net negative charge was exploited for the nonchemical modification of particles through electrostatic interaction with a cationic peptide, GRKKRRQRRRPPQ (Figure 2) [38]. This sequence is derived from the human immunodeficiency virus-1 (HIV-1) viral protein TAT (trans-activator of transcription) and is known as a cell-penetrating peptide [39][40]. Thus, the cationic peptide was used to endow heparin–protamine nanoparticles with transmembrane transport ability. The cationic peptide was adsorbed onto heparin–protamine nanoparticles, making the net charge of the particles less negative. The resultant peptide-decorated nanoparticles were loaded with model proteins, namely, β-galactosidase and RNase T1. It is noted that both of the loaded proteins have net negative charges, suggesting that those proteins interacted with the polyelectrolyte complexes through local charges in proteins. In vitro experiments revealed that the peptide-decorated nanoparticles transported proteins into cells due to the cell-penetrating ability derived from the attached peptides. Furthermore, targeted protein delivery to mouse hepatocytes was achieved in vivo with peptide-decorated nanoparticles through a hydrodynamics-based injection method.
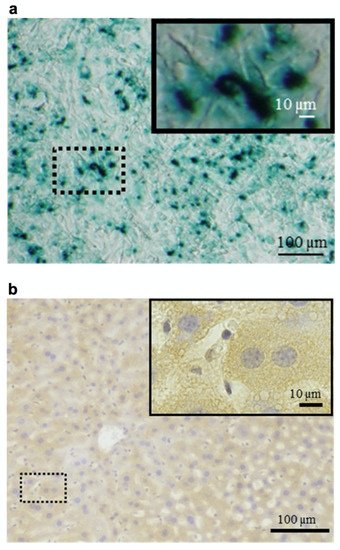
Figure 2. Protein delivery using heparin–protamine nanoparticles decorated with a cell-penetrating peptide. (a) In vitro delivery of β-galactosidase into cells. Blue cytoplasmic deposits indicate successful delivery. (b) In vivo delivery of β-galactosidase to mouse hepatocytes through hydrodynamics-based injection. Brown cytoplasmic deposits observed throughout the liver specimens indicate successful delivery.
While proteins can be loaded easily into heparin–protamine particles as described above, small-molecule drug-loading efficiency tends to be relatively low. To improve the efficiency, calcium carbonate (CaCO3) was incorporated into heparin–protamine complexes [41]. The organic–inorganic hybrid drug carriers were prepared by mixing the solution containing protamine and CO32− and the solution containing heparin and Ca2+ under particular conditions, resulting in particles with a vesicular morphology. The presence of CaCO3 increased the loading efficiency of a small-molecule drug, doxorubicin, possibly due to the presence of nanopores in the inorganic–polymer hybrid assemblies and decreased drug permeability by CaCO3. Another small-molecule drug, tariquidar, was also loaded at a low content into the hybrid nanovesicles. In addition to increased drug loading capacity, CaCO3, which has a relatively high water solubility at a low pH, endowed the system with pH sensitivity; the loaded antitumor drugs were preferentially released at lower pH. This pH sensitivity was favorable for drug delivery to tumor sites with a relatively low pH. In vitro experiments with nonresistant cells (HeLa and MCF-7) and drug-resistant cancer cells (MCF-7/ADR) showed that the dual drug-loaded nanovesicles exhibited improved tumor cell inhibitory efficiency, especially for drug-resistant cells. In a later study, a tumor-targeting ligand, biotin, was additionally introduced into hybrid nanovesicles to enhance cell uptake through biotin receptor-mediated endocytosis [42].
2.3. Chemically Modified Particles
Chemical modification is a powerful strategy to generate various functional drug carriers from heparin and protamine. To date, controlled release of small-molecule drugs, oral delivery, and improved anticancer efficacy have been achieved by using chemically modified heparin–protamine particles, as shown below.
Heparin–protamine nanocapsules were chemically crosslinked to serve as carriers of small-molecule drugs [43]. The nanocapsules were prepared by the layer-by-layer assembly of heparin and protamine on a silica template, followed by loading of the anticancer drug doxorubicin and chemical crosslinking. Chemical crosslinking prevented the premature release of loaded doxorubicin, possibly due to decreased permeability of the nanocapsule walls. In vitro experiments using MCF-7 breast cancer cells showed that the nanocapsules were readily internalized and degraded inside the cells, releasing the loaded doxorubicin and causing cancer cell death.
Bile acid-conjugated heparin–protamine nanoparticles were found to be orally available [44]; oral availability is a challenging characteristic for biomolecular nanoparticles due to biological barriers in the body [45][46][47]. After chemical conjugation with bile acid, low-molecular-weight heparin was mixed with protamine for nanoparticulate complex formation [44]. The bile acid-conjugated nanoparticles successfully attached to the enterocyte surface and were then internalized by the cells through interaction between the bile acid on the nanoparticles and the bile acid transporters of the cells. Animal experiments using nude mice revealed that orally administered nanoparticles interacted with bile acid transporters in the ileum and were taken up by epithelial cells.
For antiangiogenic therapy, a low-molecular-weight heparin–taurocholate conjugate—LHT7—which contains ~7 taurocholate groups in a heparin chain, has been developed and shown to act as an angiogenesis inhibitor [48][49][50]. Nevertheless, LHT7 showed toxicological effects including liver functional disturbances and limited anticancer effects [50]. To increase therapeutic duration while decreasing liver toxicity, PEGylated LHT7 was assembled with protamine to form nanoparticulate complexes [51]. The LHT7-containing nanoparticles exhibited improved antiangiogenic effects through the extended circulation and tumor accumulation of nanoparticles and the continued slow release of PEGylated LHT7. Notably, the nanoparticles diffused through leaky tumor blood vessels and extravasated through the blood vessels surrounding the collagen layer. A later study performed PEGylation on protamine, rather than heparin derivatives, to prevent undesirable structural changes to the heparin derivatives by the PEGylation process [52].
3. Adhesives for Cells
Heparin–protamine particles have attractive interactions with cells as well as proteins and, consequently, have been investigated as adhesives for cells.
3.1. Cell Culture
Heparin–protamine nanoparticles were used as coating materials for cell culture plastic plates to enhance the adhesion and growth of cells [53]. When the nanoparticle dispersions were applied to cell culture plates, the nanoparticles were adsorbed onto the plastic surfaces to form a stable coating layer. Adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells adhered well to the coated plates due to the adhesive properties of the heparin–protamine layer. Moreover, the heparin–protamine coating layers seemed to adsorb various heparin-binding substances from platelet-rich plasma supplemented with FGF-2, stimulating cell proliferation. Importantly, these cells maintained their multilineage potential for differentiation into adipocytes or osteoblasts. A later study demonstrated the three-dimensional culture of various human cells by using human plasma–medium gels containing heparin–protamine microparticles [54].
3.2. Cell Transplantation
Cell transplantation is a promising therapeutic strategy for tissue regeneration [55][56][57]. Nevertheless, there are still challenges, including poor survival and integration of transplanted cells in the targeted tissues. For cell transplantation, heparin–protamine microparticles were used as adhesives for the production of cell aggregates [58]. Human synovial mesenchymal stem cells formed aggregates upon mixing with the adhesive microparticles while maintaining cell viability. When injected into a cartilage defect model in the pig femoral trochlea, cell aggregates with heparin–protamine microparticles were prevented from leaking from the transplanted site. Additionally, further experiments using an osteoarthritic rabbit model suggested that the cell aggregates regenerated cartilage defects even in patients with advanced osteoarthritis, although the mechanisms mediating regeneration of cartilage and cardiomyocytes have yet to be elucidated.
Adipose-derived stromal cell aggregates with heparin–protamine particles were shown to ameliorate limb ischemia in a mouse model (Figure 3) [59]. The cell aggregates were allotransplanted into unilateral hindlimb ischemic muscles induced in adult mice by ligation of the iliac artery and hindlimb vein. Cell transplantation promoted neovascularization and prevented ischemic limb loss. Heparin–protamine particles seemed not only to induce cell aggregate formation but also to immobilize, retain, and gradually release various heparin-binding growth factors from adipose-derived stromal cells, leading to sustained vascularization.
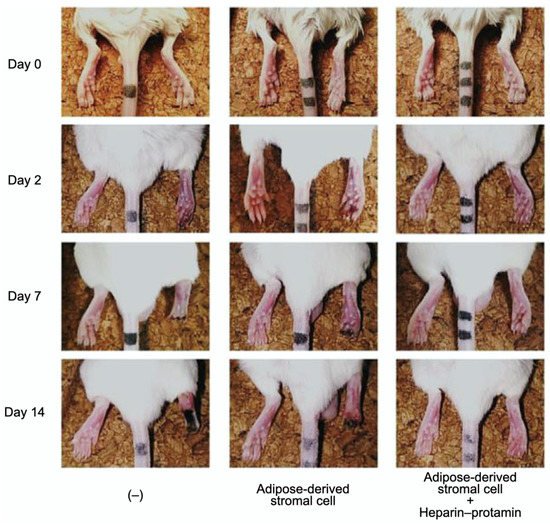
Figure 3. Amelioration of limb ischemia in a mouse model by the transplantation of adipose-derived stromal cell aggregates with heparin–protamine particles.
3.3. Skin Grafting
Skin grafting is a common technique for treating burns, chronic ulcers, and skin defects after cutaneous surgical procedures [60][61][62]. Nevertheless, skin grafts tend to suffer from stagnated revascularization, which leads to poor outcomes. It was reported that heparin–protamine particles were useful to increase the survival rate of full-thickness skin grafts [63]. Heparin–protamine particles and various growth factors from platelet-rich plasma were injected into full-thickness skin wounds created on the dorsal skin of rats, followed by full-thickness skin grafting. This therapeutic approach effectively promoted the survival rate of full-thickness skin grafts with increased blood flow and new vessel formation at the grafting site.
References
- Kunitake, T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew. Chem. Int. Ed. 1992, 31, 709–726.
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688.
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333.
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302.
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269.
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675.
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346.
- Tørring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of complex structures. Chem. Soc. Rev. 2011, 40, 5636–5646.
- Hata, Y.; Serizawa, T. Self-assembly of cellulose for creating green materials with tailor-made nanostructures. J. Mater. Chem. B 2021, 9, 3944–3966.
- Hata, Y.; Serizawa, T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull. Chem. Soc. Jpn. 2021, 94, 2279–2289.
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651.
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177.
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272.
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586.
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142.
- McMillan, J.R.; Hayes, O.G.; Winegar, P.H.; Mirkin, C.A. Protein materials engineering with DNA. Acc. Chem. Res. 2019, 52, 1939–1948.
- Stephanopoulos, N. Hybrid nanostructures from the self-assembly of proteins and DNA. Chem 2020, 6, 364–405.
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118.
- Ishihara, M.; Shaklee, P.N.; Yang, Z.; Liang, W.; Wei, Z.; Stack, R.J.; Holme, K. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology 1994, 4, 451–458.
- Ishihara, M.; Ono, K. Structure and function of heparin and heparan sulfate; heparinoid library and modification of FGF-activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233.
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331.
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927.
- Park, K.W. Protamine and protamine reactions. Int. Anesthesiol. Clin. 2004, 42, 135–145.
- Maurer, J.; Haselbach, S.; Klein, O.; Baykut, D.; Vogel, V.; Mäntele, W. Analysis of the complex formation of heparin with protamine by light scattering and analytical ultracentrifugation: Implications for blood coagulation management. J. Am. Chem. Soc. 2011, 133, 1134–1140.
- Hogwood, J.; Mulloy, B.; Gray, E. Precipitation and neutralization of heparin from different sources by protamine sulfate. Pharmaceuticals 2017, 10, 59.
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155.
- Gospodarowicz, D. Fibroblast growth factor and its involvement in developmental processes. Curr. Top. Dev. Biol. 1990, 24, 57–93.
- Akita, S.; Akino, K.; Hirano, A. Basic fibroblast growth factor in scarless wound healing. Adv. Wound Care 2013, 2, 44–49.
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508.
- Ishihara, M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trends Glycosci. Glycotechnol. 1993, 5, 343–354.
- Ishihara, M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology 1994, 4, 817–824.
- Takikawa, M.; Nakamura, S.; Ishihara, M.; Takabayashi, Y.; Fujita, M.; Hattori, H.; Kushibiki, T.; Ishihara, M. Improved angiogenesis and healing in crush syndrome by fibroblast growth factor-2-containing low-molecular-weight heparin (Fragmin)/protamine nanoparticles. J. Surg. Res. 2015, 196, 247–257.
- Kinoda, J.; Ishihara, M.; Nakamura, S.; Fujita, M.; Fukuda, K.; Sato, Y.; Yokoe, H. Protective effect of FGF-2 and low-molecular-weight heparin/protamine nanoparticles on radiation-induced healing-impaired wound repair in rats. J. Radiat. Res. 2018, 59, 27–34.
- Takabayashi, Y.; Nambu, M.; Ishihara, M.; Kuwabara, M.; Fukuda, K.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin. Cosmet. Investig. Dermatol. 2016, 9, 127–134.
- Takikawa, M.; Nakamura, S.I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 373–380.
- Takabayashi, Y.; Ishihara, M.; Sumi, Y.; Takikawa, M.; Nakamura, S.; Kiyosawa, T. Platelet-rich plasma-containing fragmin-protamine micro-nanoparticles promote epithelialization and angiogenesis in split-thickness skin graft donor sites. J. Surg. Res. 2015, 193, 483–491.
- Nakamura, S.; Ando, N.; Ishihara, M.; Sato, M. Development of novel heparin/protamine nanoparticles useful for delivery of exogenous proteins in vitro and in vivo. Nanomaterials 2020, 10, 1584.
- Brooks, H.; Lebleu, B.; Vivès, E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577.
- Torchilin, V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558.
- Gong, M.-Q.; Wu, J.-L.; Chen, B.; Zhuo, R.-X.; Cheng, S.-X. Self-assembled polymer/inorganic hybrid nanovesicles for multiple drug delivery to overcome drug resistance in cancer chemotherapy. Langmuir 2015, 31, 5115–5122.
- Gong, M.-Q.; Wu, C.; He, X.-Y.; Zong, J.-Y.; Wu, J.-L.; Zhuo, R.-X.; Cheng, S.-X. Tumor targeting synergistic drug delivery by self-assembled hybrid nanovesicles to overcome drug resistance. Pharm. Res. 2017, 34, 148–160.
- Radhakrishnan, K.; Thomas, M.B.; Pulakkat, S.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Stimuli-responsive protamine-based biodegradable nanocapsules for enhanced bioavailability and intracellular delivery of anticancer agents. J. Nanopart. Res. 2015, 17, 341.
- Park, J.; Choi, J.U.; Kim, K.; Byun, Y. Bile acid transporter mediated endocytosis of oral bile acid conjugated nanocomplex. Biomaterials 2017, 147, 145–154.
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40.
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28.
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007.
- Lee, E.; Kim, Y.-S.; Bae, S.M.; Kim, S.K.; Jin, S.; Chung, S.W.; Lee, M.; Moon, H.T.; Jeon, O.-C.; Park, R.W.; et al. Polyproline-type helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int. J. Cancer 2009, 124, 2755–2765.
- Chung, S.W.; Lee, M.; Bae, S.M.; Park, J.; Jeon, O.C.; Lee, H.S.; Choe, H.; Kim, H.S.; Lee, B.S.; Park, R.-W.; et al. Potentiation of anti-angiogenic activity of heparin by blocking the ATIII-interacting pentasaccharide unit and increasing net anionic charge. Biomaterials 2012, 33, 9070–9079.
- Alam, F.; Chung, S.W.; Hwang, S.R.; Kim, J.; Park, J.; Moon, H.T.; Byun, Y. Preliminary safety evaluation of a taurocholate-conjugated low-molecular-weight heparin derivative (LHT7): A potent angiogenesis inhibitor. J. Appl. Toxicol. 2015, 35, 104–115.
- Alam, F.; Al-Hilal, T.A.; Chung, S.W.; Park, J.; Mahmud, F.; Seo, D.; Kim, H.S.; Lee, D.S.; Byun, Y. Functionalized heparin-protamine based self-assembled nanocomplex for efficient anti-angiogenic therapy. J. Control. Release 2015, 197, 180–189.
- Park, J.; Hwang, S.R.; Choi, J.U.; Alam, F.; Byun, Y. Self-assembled nanocomplex of PEGylated protamine and heparin–suramin conjugate for accumulation at the tumor site. Int. J. Pharm. 2018, 535, 38–46.
- Kishimoto, S.; Ishihara, M.; Mori, Y.; Takikawa, M.; Hattori, H.; Nakamura, S.; Sato, T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J. Tissue Eng. Regen. Med. 2013, 7, 955–964.
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Takikawa, M.; Sumi, Y.; Nakamura, S.; Fujita, M.; Sato, T.; Kiyosawa, T. Three-dimensional culture using human plasma-medium gel with fragmin/protamine microparticles for proliferation of various human cells. Cytotechnology 2014, 66, 791–802.
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712.
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647.
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456.
- Yeo, J.E.; Nam, B.M.; Yang, W.; Jo, Y.H.; Lee, S.; Nemeno, J.G.; Kiml, B.Y.; Koh, Y.G.; Lee, J.I. Fragmin/protamine microparticle carriers as a drug repositioning strategy for cell transplantation. Transplant. Proc. 2013, 45, 3122–3126.
- Kishimoto, S.; Inoue, K.; Nakamura, S.; Hattori, H.; Ishihara, M.; Sakuma, M.; Toyoda, S.; Iwaguro, H.; Taguchi, I.; Inoue, T.; et al. Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia. Atherosclerosis 2016, 249, 132–139.
- Johnson, T.M.; Ratner, D.; Nelson, B.R. Soft tissue reconstruction with skin grafting. J. Am. Acad. Dermatol. 1992, 27, 151–165.
- Valencia, I.C.; Falabella, A.F.; Eaglstein, W.H. Skin grafting. Dermatol. Clin. 2000, 18, 521–532.
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945.
- Takabayashi, Y.; Ishihara, M.; Kuwabara, M.; Takikawa, M.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Improved survival of full-thickness skin graft with low-molecular weight heparin-protamine micro/nanoparticles including platelet-rich plasma. Ann. Plast. Surg. 2017, 78, 562–568.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
778
Revisions:
3 times
(View History)
Update Date:
16 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




