Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahesh Rachamalla | + 3920 word(s) | 3920 | 2022-03-01 04:56:57 | | | |
| 2 | Rita Xu | -25 word(s) | 3895 | 2022-03-11 03:53:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rachamalla, M. Biotechnological Innovations from Ocean. Encyclopedia. Available online: https://encyclopedia.pub/entry/20459 (accessed on 09 February 2026).
Rachamalla M. Biotechnological Innovations from Ocean. Encyclopedia. Available at: https://encyclopedia.pub/entry/20459. Accessed February 09, 2026.
Rachamalla, Mahesh. "Biotechnological Innovations from Ocean" Encyclopedia, https://encyclopedia.pub/entry/20459 (accessed February 09, 2026).
Rachamalla, M. (2022, March 10). Biotechnological Innovations from Ocean. In Encyclopedia. https://encyclopedia.pub/entry/20459
Rachamalla, Mahesh. "Biotechnological Innovations from Ocean." Encyclopedia. Web. 10 March, 2022.
Copy Citation
Marine drugs are abundant in number, comprise of a diverse range of structures with corresponding mechanisms of action, and hold promise for the discovery of new and better treatment approaches for the management of several chronic diseases. There are huge reserves of natural marine biological compounds, as 70 percent of the Earth is covered with oceans, indicating a diversity of chemical entities on the planet.
marine drugs
diabetes mellitus
cancer
cardiovascular disorders
1. Introduction
Drug molecules derived from marines sources are highly heterogenous in nature due to the abundant coverage of oceans, which thereby host the lives of a wide diversity of species [1][2]. In a pharmacological preclinical study, about 75 natural compounds that were isolated from marine organisms significantly showed biological and therapeutic activities [3][4]. The first marine drug from the cone snail peptide—namely, ziconotide, under the trade name Prialt—was approved in 2004 in the United States for the management of spinal cord injury, mediating chronic pain. Later, in 2007, another marine product named trabectedin, a sea squirt metabolite, was also approved by the European Union for the treatment of soft tissue sarcoma [5]. The drugs obtained from marine sources have displayed an exceptional potential in the management of various types of chronic diseases, including cancer, due to their potent anticancer activities [5][6][7]. These drugs have also gained the great interest of those developing new antimicrobial agents. Sponges belonging to the phylum Porifera, are the oldest and most prolific marine organisms on the planet. Demospongiae, a class of Porifera, account for 83% of the species with the highest number of bioactive compounds [8]. Another marine genus named Lendenfeldia is rich in sulfated steroids, and its metabolites possess anti-HIV, anti-inflammatory, antitumor, and antifouling activities. Drug discovery programs have significantly increased their exploration of lead molecules from marine natural products, and a higher number of bioactive products have been screened for their activity and are under development in clinical trials [9]. Terrestrial plants such as digitalis, morphine, and many other natural compounds have served as drug molecule since olden times. However, the modern pharmaceutical industry has expanded a keen interest in developing drug molecules from marine sources, as they are believed to provide more novel and potent drug compounds since they can survive under extreme conditions, such as the photodynamic and extreme temperatures, pressure, and oxidative stress of the ocean [10]. The basic scientific research in pharmacology and chemistry of marine-derived natural products mainly began in the early 1970s and has now finally begun to bear fruit due to advances in analytical and screening techniques that have fastened the drug discovery process [11]. The current entry highlights the drugs obtained from marine natural products and their role in the treatment and management of chronic diseases such as cardiovascular and neurodegenerative diseases, diabetes mellitus, and cancer. These drugs offer a look into the future of promising products that can be obtained from the sea.
The development of drugs from marine sources is a highly tedious process, as it is difficult to procure and manufacture quantities of novel drug leads from marine sources [12]. For instance, the marine sponges are highly chemically versatile in nature and act as a resource of developmental compounds, such as hemiasterlin and discodermolide, which are extracted from primitive metals found in marine habitats. Sponges are extremely treasured since they are difficult to extract, and specimens are mostly collected by hand during deep and shallow water scuba diving, but also with the help of submarines equipped with robotic arms. These techniques are highly expensive and result in an uncertain yield, thereby posing a great challenge to those developing medicines and the pharmaceutical industry. Nonetheless, the interest of researchers in marine products has remained intact, which has led to the budding of innovative solutions to overcome the challenges [13][14].
2. Identification and Isolation of Bioactive Compound from Marine Natural Extract
Natural products along with their structural analogues have contributed to pharmacotherapy throughout history, especially in the management of infectious and cancerous diseases [15][16]. However, the major challenge associated with natural products has been drug discovery, due to poor techniques of screening, isolation, characterization of the drug, and its optimization—all of which has now been overcome by advances in analytical tools such as gene mining and advanced techniques of microbial culturing, which has revitalized the interest of the pharmaceutical industry in identifying drug leads from marine sources, opening several new treatment opportunities [17][18]. The natural drugs are typically products with higher molecular mass [19][20], and they present several advantages which are discussed in this entry, yet they have several drawbacks, which have led pharmaceutical companies to reduce their efforts in the discovery of natural product-derived molecular leads [21]. The screening of natural products typically consists of an extract library mainly derived from the natural sources that may not necessarily be compatible with traditional target-based assays, making it tedious to identify bioactive compounds of interest [22]. Several tools and techniques are applied to assess whether a new molecule has been discovered or whether it is merely a rediscovery of already known compounds—a process that can be very challenging [23]. In addition, a major hurdle faced by the pharmaceutical industry is obtaining intellectual property rights for an unmodified natural product that has relevant bioactivity, as the naturally occurring compounds cannot always be patented in their native forms; however, the simpler molecules with biological activity can be patented easily [24]. The complex structures of natural products are advantageous in generating structural analogues for exploring the structure’s activity relationships and optimizing them for targeted mechanism of action [25]. The modern techniques—which include genome mining, genome engineering, and advances in analytical procedures and systems of cultivation—have led to an increased emphasis on drug development from marine sources, as they have helped overcome many of the major challenges that were being faced by the researchers—techniques that have proven to be promising [26][27].
The application of advanced analytical techniques begins with the screening of crude drug extracts, followed by further isolation and identification of a bioactive molecule that is fractionated to obtain the active moiety from the natural product [28]. The isolation of a bioactive compound is a laborious task and is highly challenging. The molecule isolated is run through the extract libraries and further exposed to high throughput screening so that the crude extract can be pre-fractionated into subfractions that are more suitable for the system that handles automated liquids [3]. The methods of fractionation can be altered to obtain preferential subfractions with active compound drugs that are alike in nature. This process can increase the number of hits compared with the compounds obtained from crude extracts, which enables more efficient and promising hits [6]. An advance in the instrumentation used in analytical methods, combined with advanced computational approaches, can lead to isolation of possible analogue structures of natural products [29]. The precise information about the metabolic composition of the crude marine extract can be obtained through metabolomics, which helps in prioritizing the isolation of a compound and its dereplication to annotate the structural analogues of the newly derived product [30] (Figure 1).
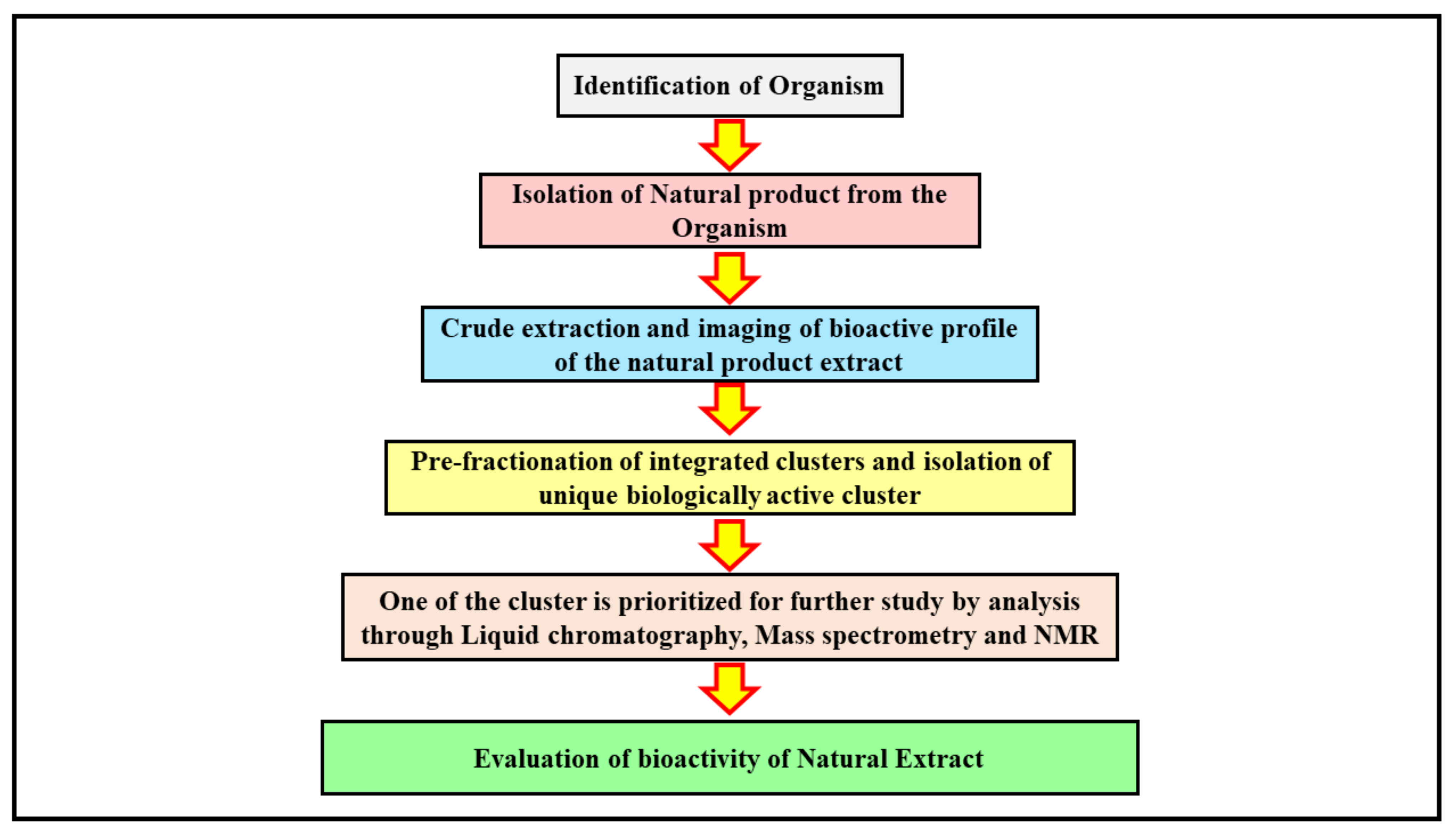
Figure 1. The figure illustrates the process of isolation of a bioactive molecule from a marine source. The identification of a specific organism is a first step in determining the bioactive lead moiety. Once the marine source with a desired therapeutic effect is discovered, the natural products within it are obtained by extraction processes. The crude extract collected at the end is further studied using imaging techniques to understand its bioactive profile. The integrated clusters of the bioactive compound in the crude extract solution are further pre-fractionated to obtain unique clusters that are biologically active. The active clusters are analyzed through advanced analytical techniques such as NMR, mass spectroscopy, and liquid chromatography for their structure elucidation and bioactivity determination, and the desired cluster is carried forward for further studies. The bioactive lead molecule selected is evaluated for its therapeutic activity through pre-clinical and further clinical trial studies.
3. Marine Drugs in the Management of Cancer
Cancer is a chronic disease and can be found in almost all multicellular organisms. The disease is strongly associated with aging because, with increasing age, mutations within the somatic cells accumulate and promote unregulated growth and invasion of dysfunctional cells, which leads to altered functions in the body and works against the organism’s health. Apart from the uncontrolled cellular growth, this disease also displays genetic instability, evasion of growth suppressors, immortality of replication, resistance to cell death, angiogenesis initiation, energy metabolism reprogramming, prevention of immune destruction, tumor-derived inflammation, and metastasis [31][32][33]. Advanced research and technology combined with efforts to determine and understand the hallmarks of cancer have led to improvement in the clinical outcomes of cancer patients by developing newer and more novel diagnostic techniques and therapeutic medications. The higher costs of these therapies and the sometimes extension of patient survival by just few months pose a major challenge to ongoing treatment, thereby provoking a dire need to identify promising new therapies for the management of this life threating disease. There are two general classes of cancer therapies: cancer therapies derived from natural compounds and cancer therapies derived from synthetic compounds—each further subcategorized into small molecules or low molecular weight substances that elicit biological responses by entering cells readily and biologics or large molecular weight substances such as ribonucleic acid (RNA) or monoclonal antibodies that are penetrated across cells with the help of delivery systems [34]. The majority of the cancer treatment drugs are naturally derived substances. For instance, the most primarily used chemotherapeutic drugs for the management of prostrate, breast, and other cancers—namely, docetaxel and paclitaxel—are derived from the taxanes plant. Cabazitaxel, another naturally derived anticancer compound was derived by chemical diversification of taxanes [35]. Factually, most of the anticancer drugs derived from natural resources are derived from terrestrial ecosystems, and about 100,000 compounds have been isolated from plants alone. About 99% of the total living space on earth is deep ocean, and oceans are where80% of the entire species in the world live. In the recent years, the interest of researchers has progressively focused on the marine environment, and researchers have successfully isolated over 2000 compounds over the past three decades [36]. The richness in species of the ocean and its extraordinary diversity with a large temperature and pressure tolerance window, presence of variety of chemicals and metals, saline nature, low to bright light, and allelopathic defenses has attracted the pharmaceutical industries towards the ocean, despite the small number of compounds isolated from it to date. Marine sources are believed to have treasurable therapeutic potential based on the unique dwelling inhabitant and hence the ocean is being explored for its hidden potential. Its noteworthy that the products derived from the ocean are extremely potent and act through multiple molecular pathways and collectively have an ability to target different hallmarks of cancer [37]. Some of the anticancer compounds isolated from marine sources are listed below.
Over 1000 compounds have been isolated from marine sources and are being tested for their activity in preclinical studies; 23 marine derived compounds are under clinical trials between phases I to III, and 7have been approved for marketing. Four compounds out of the total number of marine-derived molecules—namely, trabectedin, cytarabine, eribulin mesylate, and brentuximab vedotin, a conjugated antibody—are being used clinically for their anticancer properties [38].
3.1. Cytarabine (Cytosar)
Cytarabine is the debutant lead molecule that has been isolated from the ocean for the management of cancer. Cytarabine was developed by the synthesis of analogs of natural arabino nucleosides and cytosine arabinose from the Caribbean sponge Cryptotethya crypta. The chemical structure of this anticancer compound has been found to be related to Spong uridine and Spong thymidine, which are natural products isolated from the marine sponge Tectitethya cripta. Cytarabine acts by rapidly converting into its respective triphosphates arabinonucleoside by phosphorylation in a sequential manner. It is an antimetabolite drug with a structure that is sufficiently similar to natural metabolites of the body and acts by interfering with their functioning and hence preserving normal cellular metabolism [39]. The triphosphocytarabine formed upon phosphorylation becomes a substrate for DNA polymerase and subsequently is amalgamated in place of cytosine within the DNA. The arabinose is implanted instead of deoxyribose upon binding of DNA with cytarabine triphosphate and promotes elongation of the DNA strain by preventing phosphodiester bonding between the two pentose sugars, thereby prohibiting synthesis of DNA and hampering abnormal cellular growth. This drug was first approved for its clinical applications in 1969 and has been used in the management of wide array of leukemias, such as non-Hodgkin’s lymphoma, acute lymphocytic leukemia, chronic myelogenous leukemia, etc. Cytarabine has been claimed to be the foremost example of a commercially available marine-derived drug, even though the molecule itself is not a natural product but a structural analog [40]. The use of adenine arabinose analogs has also been noted in the development of vidarabine, an antiviral drug used in the management of varicella zoster and herpes simplex virus [41]. Furthermore, another antiviral drug, azidothymidine, has also reportedly been found to be extremely effective in the management of acquired immune deficiency syndrome (AIDS) by blocking the activity of the reverse transcriptase enzyme of the virus, which is highly crucial for replication of the human immune virus (HIV).
3.2. Trabectedin
Trabectedin was identified as one of the most abundant structurally related alkaloids that has been isolated from the Caribbean ascidian Ecteinascidia turbinate. The drug was first isolated with a very complex procedure in 1996. This molecule acts as an alkylator of DNA but differs from the usual alkylating agents. Trabectedin binds with the guanine residues of the double helix DNA and generates specific sequences, causing bending of strands in a direction opposite to the site of alkylation. These trabectedin abducts arrest the activity of RNA polymerase II and prevent the transcription of DNA, thereby preventing abnormal cellular growth. The most prominent effect of this drug is the inhibition of transcription of MDR1 genes, which are chiefly responsible for producing P glycoproteins and initiating the detoxification processes in the cells. The drug also successfully prevents the repair of DNA lesions due to the rest of RNA polymerase II, thereby producing a significant impact on the tumor microenvironment. Trabectedin also activates caspase 8 protein, which further induces apoptosis in macrophages and monocytes, thereby prohibiting the release of inflammatory mediators and the growth of angiogenic factors and preventing metastasis. Trabectedin acts via DNA alkylation by binding to the guanine residues, further generating exclusive sequences that cause bending of the double helix strands in a direction opposite to that of alkylation and differing greatly from other alkylating agents. The action of RNA polymerase II is arrested with simultaneous DNA transcription prohibition, hence preventing the abnormal growth of the cells. The drug prominently acts by inhibiting MDR1 genes transcription, which chiefly indulging in the synthesis of P glycoproteins, thereby stimulating the process of cellular detoxification [42]. The repair of the lesions in DNA is also counteracted by this drug due to RNA polymerase II arrest, hence producing a significant effect in the deterioration of the environment of the tumor.
3.3. Eribulin Mesylate
Eribulin is a mesylate salt prototype of high molecular weight halichondrin B, obtained from a class of halichondrins, a progression of macrocyclic polyethersthathave anticancer activity. The drug is obtained from Halichondria okadai sponges found on the coast of the Miura Peninsula in Japan. The macrocyclic ring of halichondrin B is responsible for the anticancer activity, as determined by the structure–activity relationship. This drug is believed to be the most complex drug synthesized. This naturally derived potent antimitotic drug mainly acts by inhibiting the microtubule. Tubulin is the ultimate target of this compound, and it prevents polymerization by binding to it and further arresting the microtubular extension. This irreversibly blocks the mitosis of the cells, and its prolongation finally induces apoptosis-mediated cell death. The drug eribulin mesylate was approved in 2010 by the FDA for the management of advanced metastatic breast cancer, and it was approved as second-line treatment for liposarcoma therapy in 2016 [43].
3.4. Brentuximab Vedotin
In 1972, anticancer activity was discovered from the extract of Dolabella auriculria, a gastropod mollusk found in the Indian Ocean. After 15 years of continuous research, the peptides—namely, dolastatins—were identified as being the chief active compounds that solely possess potent antiproliferative activity against tumor cells [43]. These peptides led to microtubule blockage and polymerization, consequently preventing rapid tumor cell division and hence prohibiting the growth and proliferation of tumor cells in the body. Brentuximab vedotin has an antibody drug conjugate structure and is mainly developed with a dolastatin10-derived natural product molecule, monomethylauristatin E, isolated from the mollusk Dolabella auricularia. In 2012, Adcetris had been approved for the treatment and management of Hodgkin’s lymphoma, and monomethylauristatin E had also been studied in several clinical trials based on its ability to form complexes with antibodies and proteins on the membrane. Another analogue—namely, glembatumumabvedotin—has also been recognized for its activity in the management of melanomas, especially those with metastatic breast cancer, due to its association with transmembrane glycoproteins [44]. Polatuzumabvedotin and pinatuzumabvedotin are also being evaluated for their activity in the management of lymphomas and leukemias, due to their direct antibody-directed action on CD 22 and CD 79b proteins (Table 1).
Table 1. The below table lists the marine drugs used in cancer treatment and their respective structures.
| Name | Structure |
|---|---|
| Cytarabine | 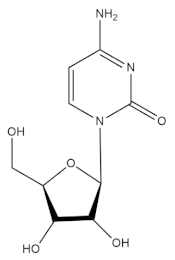 |
| Trabectedin | 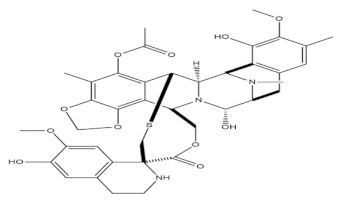 |
| Eribulin Mesylate | 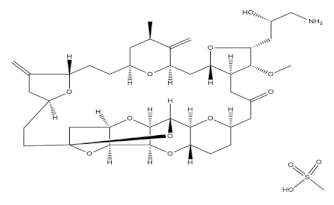 |
| Brentuximab vedotin | 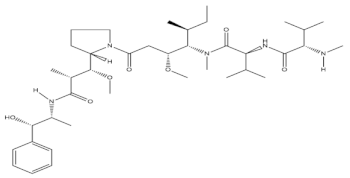 |
4. Marine Drugs in the Management of Diabetes Mellitus
Diabetes mellitus is a chronic metabolic disease that is mainly characterized by blood sugar level elevation and abnormal metabolism of sugar, and it contributes in a major way to mortality and morbidity rates across several developing and developed nations, including India. This disease includes a defect in the functioning of insulin, including its secretion and site of action. The destruction of other microstructures such as neurons, nephrons, and retina depicts the severity of the disease, highlighting its role in affecting nerves, kidney, and eyes. Several cardiovascular disorders and other conditions also occur upon the emergence of diabetes [45]. An increase in reactive oxygen species and oxidative stress is considered as playing a crucial role in the development of diabetes and its associated complications [46]. Food acts as a major source of energy in the form of sugar and helps in maintaining the physiological functioning of the millions of cells in the body. The body regulates sugar by moving it through the cell membrane via two mechanisms: a receptor, which acts as a door, and insulin, which targets the receptors. Type 1 diabetes is a hyperglycemic condition with a defect in insulin, while type 2 diabetes is mainly characterized by increased levels of sugar, mostly due to a defect in receptors [45]. The prevalence of type 2 diabetes is higher globally in comparison with type 1diabetes. It has been estimated that more than 20 million people worldwide have been diagnosed with type 1diabetes, with a predicted annual increase of 2% to 5% every year in several countries. However, type 2 diabetes accounts for approximately 90% to 95% of diabetic cases globally. In the year 2011, over 280 million people were estimated to be affected by type 2 diabetes and the number is assumed to rise to up to 500 million by 2030 [47]. It has become necessary to adopt preventive measures to reduce the burden of this disease on the health and economy of a country. Several synthetic drugs are available in the market to treat this disease, but the medications are not fully effective and are also costly. Moreover, continued use of these drugs can lead to undesirable adverse reactions in the patients. Patients suffering with type 1 diabetes are solely dependent on external insulin injections for their survival and for maintaining a normal life, but it is not comfortable to inject insulin daily. However, type 2 diabetes can be initially managed or controlled by modifications in lifestyle and diet, but type 2 diabetes often requires treatment with oral antidiabetic drugs in the disease’s later stages, and at the end, treatment typically requires insulin injections, which is the most severe scenario. Antioxidants and immune therapy, islet therapy, inhibitors of alpha glucosidase, and other antidiabetic drugs are some of the available therapies to control and manage type 2 diabetes mellitus, but a wide range of side effects also come alongside, which has led to the continued development of novel preventive and regenerative therapies for preventing deficiencies in beta cells mass and for prolong the earlier stage of this disease. Marine sources are being explored due to their promising potential as therapeutic agents in the management of various medical disorders so that they can be employed as a novel or adjuvant therapies. Several marine drugs have been identified in recent years for their exceptional potential for treating or curing diseases. Algae and fish have acted as chief sources of several peptide molecules that possess lipid lowering, anticancer, and anticoagulation properties [48]. In addition, principal antioxidants such as phenolics, carotenoids, and omega 3 fatty acids are also derived from marine seaweeds, crustaceans and fish oil, and bacteria, respectively.
Over 500 marine and freshwater cyanobacteria have been studied for anti-glucosidase and anti-amylase activity, and 38 interesting candidates have also been determined to be fruitful in the management of diabetes [49]. A marine sponge-related bacterium known as Coralliphaga has been found to possess major activity in polysaccharide degradation and in the processing of glycolipids and glycoproteins, as it produces a number of glucosidase inhibitors, presenting it as a good target for development in the treatment of diabetes, as well as obesity. Strains of Streptomyces bacteria such as Streptomyces corchorusii subspecies rhodomarinus presented fascinating antidiabetic properties by inhibiting the activity of enzyme amylase, while other species of a similar strain led to the production of two novel compounds having N-acetyl-glucosaminidase inhibition properties—namely, Pyrostatins A and B [50]. In addition, to the antidiabetic activity of bacteria, cyanobacteria, and actinomycetes, marine fungi have also been screened to assess whether they might have antidiabetic action. A protein tyrosine phosphatase (PTP1B) inhibitor—namely, aquastatin B—has been obtained from the marine fungus Cosmospora species SF-5060, which was isolated from the sediment collected from inter-tides at Gejae Island in Korea [51]. Photosynthetic eukaryotic microalgae, which form a major part of freshwater and marine phytoplankton, have also been confirmed to have significant activity in the management of diabetes mellitus [52][53].
Recent biotechnological advances in aquatic technology have successfully identified promising antidiabetic agents in microalgal species, by virtue of their anti-glycation function. The green microalgal species named Chlorella and the diatom Nitzschia laevis have been found to have maximum inhibitory effects against the formation of total advanced glycated end products (AGEs)—specifically, N-carboxymethyllysine and pentosidine [54]. The presence of carotenoids such as neoxanthin, antheraxanthin, violaxanthin, and lutein account for the strong AGEs inhibitory activity of Chlorella, while linoleic acid, eicosapentaenoic acid, and arachidonic fatty acids have presented similar bioactivity in Nitzschia laevis. The different extracts of these microalgae—mainly Chlorella zofingiensis—were tested for their antiglycation activity, and it was determined that the extracts rich in astaxanthin possessed the highest antiglycative and antioxidant activity and hence can be used as a food supplement for the prevention of diabetes in patients [55]. Three strains of microalgae—namely, Chlorella protothecoides, Chlorella zofingiensis, and the diatom N. laevis—have been evaluated to possess protective action against the exogenous and endogenous AGEs in an ARPE-19 cell-based model due to the presence of nutritional ingredients such as carotenoids and omega 3 fatty acids within them.
Therefore, it can be concluded that marine products and byproducts, if explored judicially, can be the source of several promising and novel lead molecules in the treatment and management of diabetes (Table 2).
Table 2. The below table lists the marine drugs used in diabetes mellitus and their respective structures.
| Name | Structure |
|---|---|
| Pyrostatins A and B | 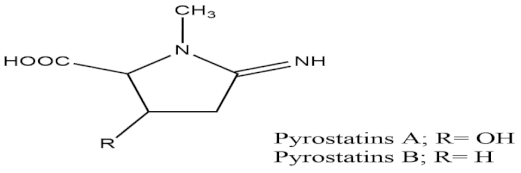 |
| N-carboxymethyllysine | 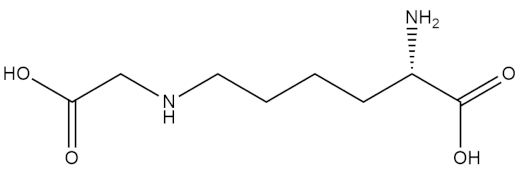 |
| Pentosidine | 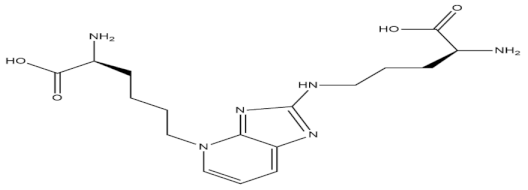 |
| neoxanthin | 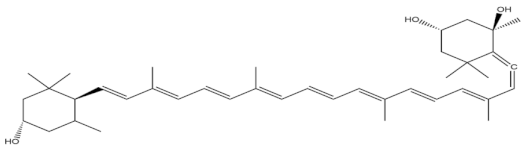 |
| antheraxanthin | 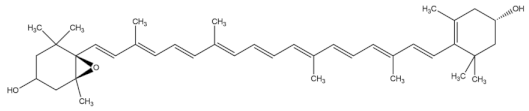 |
| violaxanthin | 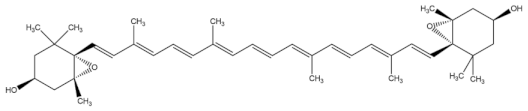 |
| Lutein | 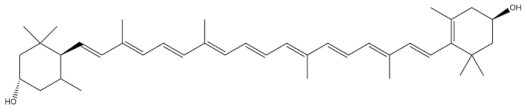 |
References
- Jiménez, C. Marine Natural Productsin Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961.
- Newman, D.J.; Cragg, G.M. Natural Productsas Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discov. Today 2016, 21, 204–207.
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sourcesas a Tool for Functional Food Development. Front. Mar. Sci. 2022, 76, 832957.
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2009, 26, 170–244.
- Newman, D.J.; Cragg, G.M. Drugs and Drug Candidates from Marine Sources: An Assessment of the Current “StateofPlay”. Planta Med. 2016, 82, 775–789.
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. De Chim. 2019, 70, 3103–3107.
- Altmann, K.H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia 2017, 71, 646–652.
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69.
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347.
- Moumbock, A.F.A.; Li, J.; Mishra, P.; Gao, M.; Günther, S. Current Computational Methods for Predicting Protein Interactions of Natural Products. Comput. Struct. Biotechnol. J. 2019, 17, 1367.
- Moumbock, A.F.A.; Simoben, C.V.; Wessjohann, L.; Sippl, W.; Günther, S.; Ntie-Kang, F. Computational studies and biosynthesis of natural products with promising anticancer properties. In Natural Products and Cancer Drug Discovery; InTech Open: London, UK, 2017.
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus towards in Vitro Models and Adverse Outcome Pathways (AOP). Front. Pharmacol. 2021, 12, 2157.
- Walsh, C.T.; Fischbach, M.A. Natural Products Version 2.0: Connecting Genes to Molecules. J. Am. Chem. Soc. 2010, 132, 2469–2493.
- Grover, M.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Virmani, T.; Rachamalla, M.; Farasani, A.; Chigurupati, S.; Alsubayiel, A.; et al. In Vitro Phytochemical Screening, Cytotoxicity Studies of Curcuma Longa Extracts with Isolation and Characterisation of Their Isolated Compounds. Molecules 2021, 26, 7509.
- Puppala, E.R.; Jain, S.; Saha, P.; Rachamalla, M.; Syamprasad, N.P.; Yalamarthi, S.S.; Abubakar, M.; Chaudhary, A.; Chamundeswari, D.; Murty, U.S.N.; et al. Perillyl Alcohol Attenuates Rheumatoid Arthritis via Regulating TLR4/NF-Κ Band Keap1/Nrf2 Signaling Pathways: A Comprehensive Study On in-Vitro and in-Vivo Experimental Models. Phytomedicine 2022, 97, 153926.
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug Development from Marine Natural Products. Nat. Rev. Drug Discov. 2009, 8, 69–85.
- Dinarvand, M.; Spain, M. Identification of Bioactive Compounds from Marine Natural Products and Exploration of Structure—Activity Relationships (Sar). Antibiotics 2021, 10, 337.
- Pereira, F.; Aires-de-Sousa, J. Computational Methodologies in the Exploration of Marine Natural Product Leads. Mar. Drugs 2018, 16, 236.
- Di Masi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ. 2016, 47, 20–33.
- Nantasenamat, C.; Prachayasittikul, V. Maximizing Computational Tools for Successful Drug Discovery. Expert Opin. Drug Discov. 2015, 10, 321–329.
- Gasteiger, J. Chemoinformatics: Achievements and Challenges, a Personal View. Molecules 2016, 21, 151.
- Mueller, R.; Dawson, E.S.; Meiler, J.; Rodriguez, A.L.; Chauder, B.A.; Bates, B.S.; Felts, A.S.; Lamb, J.P.; Menon, U.N.; Jadhav, S.B.; et al. Discovery of (2-(2-Benzoxazoyl Amino)-4-Aryl-5-Cyanopyrimidine MGlu 5NAMs: From Artificial Neural Network Virtual Screen to in Vivo Tool Compound. Chem. Med. Chem 2012, 7, 406.
- Leelananda, S.P.; Lindert, S. Computational Methods in Drug Discovery. BeilsteinJ. Org. Chem. 2016, 12, 2694–2718.
- Katsila, T.; Spyroulias, G.A.; Patrinos, G.P.; Matsoukas, M.T. Computational Approaches in Target Identification and Drug Discovery. Comput. Struct. Biotechnol. J. 2016, 14, 177–184.
- Rodriguez, A.L.; Grier, M.D.; Jones, C.K.; Herman, E.J.; Kane, A.S.; Smith, R.L.; Williams, R.; Zhou, Y.; Marlo, J.E.; Days, E.L.; et al. Discovery of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype5 Reveals Chemical and Functional Diversity and in Vivo Activity in Rat Behavioral Models of Anxiolytic and Antipsychotic Activity. Mol. Pharmacol. 2010, 78, 1105–1123.
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to Speed up the Natural Products Discovery Process. Nat. Prod. Rep. 2015, 32, 779–810.
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 310.
- Vijayakrishnan, R. Structure-Based Drug Design and Modern Medicine. J. Postgrad. Med. 2009, 55, 310.
- Talele, T.; Khedkar, S.; Rigby, A. Successful Applications of Computer Aided Drug Discovery: Moving Drugs from Concept to the Clinic. Curr. Top. Med. Chem. 2010, 10, 127–141.
- Jones, P.A.; Baylin, S.B. The Epigenomics of Cancer. Cell 2007, 128, 683–692.
- Chauthe, S.K.; Mahajan, S.; Rachamalla, M.; Tikoo, K.; Singh, I.P. Synthesis and Evaluation of Linear Furanocoumarinsas Potential Anti-Breast and Anti-Prostate Cancer Agents. Med. Chem. Res. 2015, 24, 2476–2484.
- Varun, K.; Mahesh, R.; Prajwal, N.L.; Khatik, G.T.; Sangamwar, A.; Kulbhushan, T.A.; Nair, V. Design and Synthesis of Optically Pure3-Aryl-6-Methyl-2-Thioxotetrahydropyrimidin-4(1H)-Ones as Anti-ProstateCancerAgents. RSC Adv. 2014, 4, 37868–37877.
- Lujambio, A.; Lowe, S.W. The Microcosmos of Cancer. Nature 2012, 482, 347–355.
- Bouchet, B.P.; Galmarini, C.M. Cabazitaxel, a New Taxane with Favorable Properties. Drugs Today 2010, 46, 735–742.
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of New Marine Bioactive Compounds An Overview of the Last Decade and Future Steps for Bioprospecting. Mar. Drugs 2011, 9, 1860–1886.
- Jensen, P.R.; Fenical, W. Marine microorganisms and drug discovery: Current status and future potential. In Drugs from the Sea; Karger: Basel, Switzerland, 2000; pp. 6–29.
- Dayanidhi, D.L.; Thomas, B.C.; Osterberg, J.S.; Vuong, M.; Vargas, G.; Kwartler, S.K.; Schmaltz, E.; Dunphy-Daly, M.M.; Schultz, T.F.; Rittschof, D.; et al. Exploring the Diversity of the Marine Environment for New Anti-Cancer Compounds. Front. Mar. Sci. 2021, 7, 1184.
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-Derived Drugs: Recent Advances in Cancer Therapy and Immune Signaling. Biomed. Pharmacother. 2021, 134, 111091.
- Patel, R.S.; Rachamalla, M.; Chary, N.R.; Shera, F.Y.; Tikoo, K.; Jena, G. Cytarabine Induced Cerebellar Neuronal Damage in Juvenile Rat: Correlating Neurobehavioral Performance with Cellular and Genetic Alterations. Toxicology 2012, 293, 41–52.
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: The First Two Decades of XXI Century. Mar. Drugs 2020, 18, 20.
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2020. Mar. Drugs 2020, 18, 643.
- Jimenez, P.C.; Wilke, D.V.; Costa-Lotufo, L.V. Marine Drugs for Cancer: Surfacing Biotechnological Innovations from the Oceans. Clinics 2018, 73, 42.
- Van de Donk, N.W.C.J.; Dhimolea, E. Brentuximab Vedotin. mAbs 2012, 4, 458–465.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, 62–67.
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular Mechanisms of ROS Production and Oxidative Stress in Diabetes. Biochem. J. 2016, 473, 4527–4550.
- Barde, S.R.; Sakhare, R.S.; Kanthale, S.B.; Chandak, P.G.; Jamkhande, P.G. Marine Bioactive Agents: A Short Review on New Marine Antidiabetic Compounds. Asian Pac. J. Trop. Dis. 2015, 5, 209–213.
- Skyler, J.S. Diabetes Mellitus: Pathogenesis and Treatment Strategies. J. Med. Chem. 2004, 47, 4113–4117.
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056–1100.
- Imada, C. Enzyme Inhibitors and Other Bioactive Compounds from Marine Actinomycetes. Antonie Leeuwenhoek 2005, 87, 59–63.
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive Compounds from Marine Bacteria and Fungi: Mini review. Microb. Biotechnol. 2010, 3, 544–563.
- Carotenuto, Y.; Esposito, F.; Pisano, F.; Lauritano, C.; Perna, M.; Miralto, A.; Ianora, A. Multi-Generation Cultivation of the Copepod Calanus Helgolandicusina Re-Circulating System. J. Exp. Mar. Biol. Ecol. 2012, 418, 46–58.
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde Suppression of Copepod Recruitmentin Blooms of a Ubiquitous Planktonic Diatom. Nature 2004, 429, 403–407.
- Sun, Z.; Peng, X.; Liu, J.; Fan, K.W.; Wang, M.; Chen, F. Inhibitory Effects of Microalgal Extracts on the Formation of Advanced Glycation Endproducts (AGEs). Food Chem. 2010, 120, 261–267.
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Astaxanthin Is Responsible for Antiglycoxidative Properties of Microalga Chlorella zofingiensis. Food Chem. 2011, 126, 1629–1635.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
966
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




