Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Julio Villena | + 2049 word(s) | 2049 | 2022-02-14 04:37:29 | | | |
| 2 | Dean Liu | -9 word(s) | 2040 | 2022-03-10 08:01:32 | | | | |
| 3 | Dean Liu | -23 word(s) | 2017 | 2022-03-11 09:25:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Villena, J. Immunomodulation Potential of Probiotics. Encyclopedia. Available online: https://encyclopedia.pub/entry/20403 (accessed on 07 February 2026).
Villena J. Immunomodulation Potential of Probiotics. Encyclopedia. Available at: https://encyclopedia.pub/entry/20403. Accessed February 07, 2026.
Villena, Julio. "Immunomodulation Potential of Probiotics" Encyclopedia, https://encyclopedia.pub/entry/20403 (accessed February 07, 2026).
Villena, J. (2022, March 09). Immunomodulation Potential of Probiotics. In Encyclopedia. https://encyclopedia.pub/entry/20403
Villena, Julio. "Immunomodulation Potential of Probiotics." Encyclopedia. Web. 09 March, 2022.
Copy Citation
The use of probiotics in livestock has been suggested to significantly improve their health, immunity, growth performance, nutritional digestibility, and intestinal microbial balance. Furthermore, it was reported that the use of probiotics in animals was helpful in equilibrating their beneficial microbial population and microbial turnover via stimulating the host immune response through specific secretions and competitive exclusion of potentially pathogenic bacteria in the digestive tract.
livestock
healthy growth strategy
probiotics
immunoregulatory effects of probiotics
1. Introduction
Antimicrobial resistance represents a global health problem that contributes to tens of thousands of deaths per year. Furthermore, the global demand for meat and dairy consumption is increasing at a rapid and unprecedented rate [1]. To fulfill this demand, many countries are shifting to intensive livestock production systems that use antimicrobial (AM) drugs to keep animals healthy and increase their development and productivity [2][3]. For example, Van Boeckel et al. (2015) found that between 2010 and 2030, the global consumption of AM agent for livestock industry increased by 67%, while on the other hand, the increase in AM agent consumption in the BRICS countries (Brazil, Russia, India, China, South Africa) will be 67%. Furthermore, Denmark was the foremost nation to report authorized antimicrobial agent manufacturing/sales data in 1996, under the name of Danish Integrated Additive Manufacturing Resistance Monitoring and Research Program (DANMAP). In 2011, the European Medicines Agency Surveillance of Veterinary Consumption group (ESVAC) published the first report on veterinary AM sales in eight countries (Czech Republic, Denmark, Finland, France, Netherlands, Norway, Sweden, UK) since 2005. The latest 2017 report provides an overview of AM sales across all EU countries. Furthermore, North American countries and Canada began collecting sales data for AM resistance monitoring in 2008 for the Canadian Comprehensive Program (CIPARS), which reports AM resistance and AM use. In Asia, Japan was the first country to launch the Japan Veterinary AM Monitoring System (JVARM) to report AM agent use [4]. In addition, current global trends in the use of AM agents in livestock animal feeds were represented in Figure 1. Therefore, the establishment of AM-free feeding system by using probiotics has been required for secure and healthy livestock production. The most commonly used probiotics in livestock are the strains of lactic acid bacteria (LAB) and Bifidobacterium [5]. In addition, gastrointestinal tract (GI) infections in livestock are considered a major global problem, with a negative economic impact on livestock farmers [6]. In this regard, the likelihood of using feed supplements to attain a healthier animal, welfare, and yield by manipulating the gut microbiota has received considerable attention over the past 30 years. Antibiotics have been applied widely to prevent and treat GI infection in livestock; however, the random uses of antibiotics in livestock are responsible for the development of antibiotic resistance, which has a long-lasting effect on the human body, as well as the destruction of gut microflora [7][8][9]. Probiotics might be used as a potential alternative therapy to treat gastrointestinal tract disorders and to enhance the endogenous immune function of the host (Figure 1).
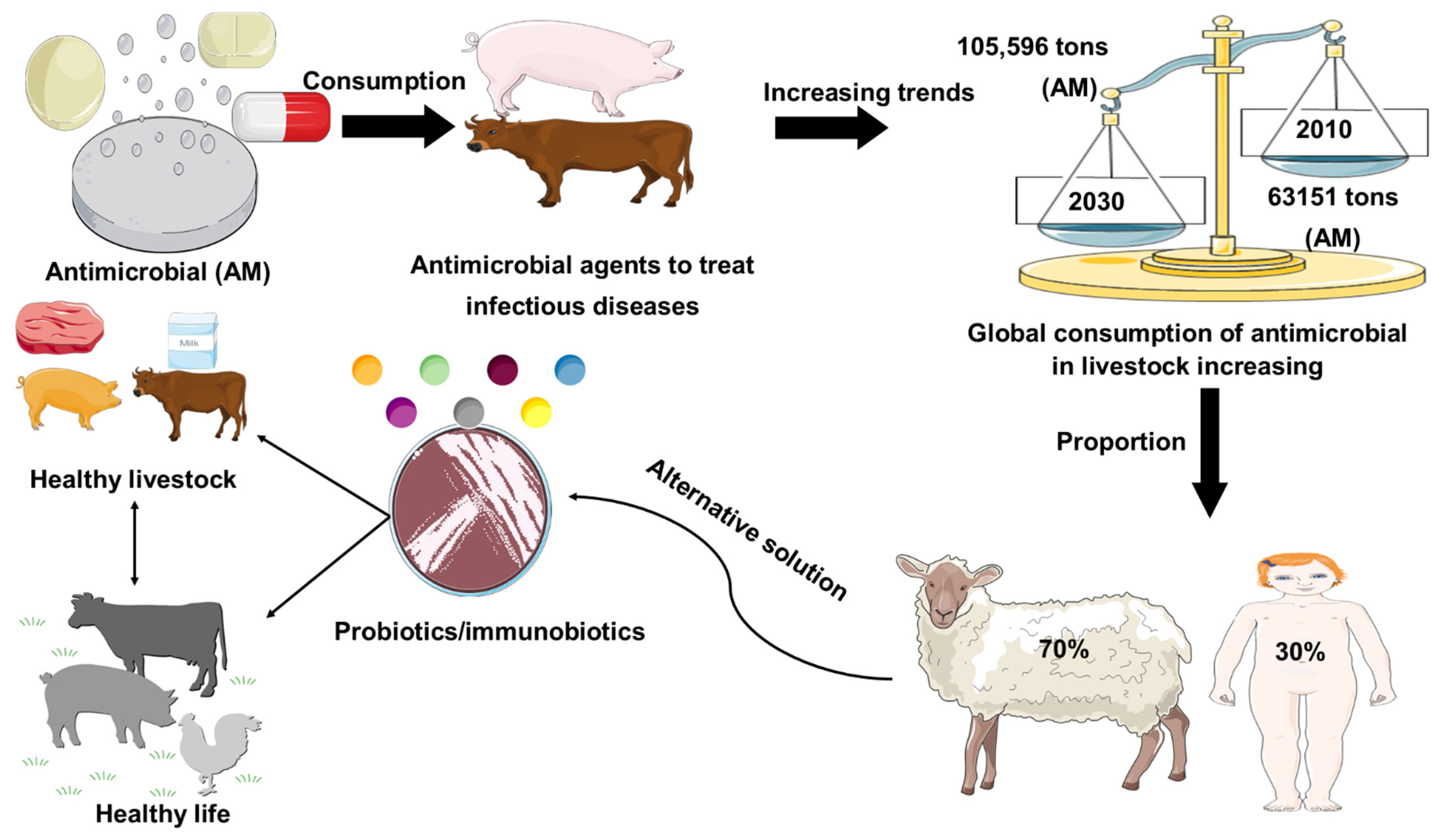
Figure 1. Role of probiotics in livestock healthy growth strategy. Global consumption of AM (AM) in livestock production was estimated in 2010 and is projected to rise by 67%, by 2030. Global increase (67%) in AM consumption is due to the growing number of animals raised for meat and milk production. Probiotics used as a safer alternative to conventional antibiotic drug therapy.
Numerous probiotics might be used to improve the performance of ruminant and pig animals. Numerous studies have demonstrated that probiotics can exert an AM effect against pathogens and improve animal health, as well as productivity [10][11]. Earlier, the team established a porcine intestinal epithelial (PIE) cell line and demonstrated that PIE cells are a useful in vitro tool for the selection of immunomodulatory LAB (immunobiotic LAB). Furthermore, the team has demonstrated that the in vitro and in vivo immunobiotic LAB is a good alternative to improve resistance against GI pathogens in the porcine host. Additionally, the laboratory has shown that the probiotic Lactobacillus with immunoregulatory functions can beneficially modulate the immune response in the gut through controlling the functions of PIE cells [10][11][12][13][14][15]. This contrasts with previous studies that recommend the modulation of gut microbiota and piglet immunity via appropriate probiotic strains, which will lead to better growth performance. Therefore, it is necessary to establish a non-toxic feeding system and a food safety system to ensure the safe and healthy production of animal husbandry. A study suggested that the probiotic-supplemented diet significantly improved the health status, growth performance, and intestinal morphology in pigs [16]. Similarly, it was suggested that the multi-species probiotic diet has excellent potential to endorse the growth performance and healthy status of pigs via modulation of gut microbiota [17].
2. Application of Probiotics in Livestock Production
In recent decades, some studies were conducted to illustrate the new scope in the field of probiotics and to discover the potential probiotic microbes. According to Sun et al., (2021) multi-species probiotics consisting of L. acidophilus, L. casei, B. thermophilum, and E. faecium were successfully used to reduce the diarrhea caused by enterotoxigenic E. coli (ETEC) F18+ in newly weaned pig [18]. In addition, multi-species probiotics were helpful in enhancing growth performance through a reduction in intestinal inflammation, oxidative stress, and morphological damages. Sobrino et al. (2021) attempted to study AM substitutes in pig production. They used Ligilactobacillus salivarius strain retrieved from sow’s milk and fed it to pregnant sows and piglets. The results suggested that there was a notable reduction in the presence of antibiotic-resistant Lactobacillus, which became apparent in the treatment group [19]. In recent studies, it was suggested that Prevotella exerted positive consequences in pig production by enhancing growth performance and immune response [20][21][22][23]. The Lactobacillus, Escherichia, Shigella, and Bacteroides dominate the small intestine microbiota, while on the other hand, the Prevotella dominates the large intestinal microbiota during the newborn stage. Furthermore, the Prevotella dominates the pig’s small and large intestines after weaning [24]. Additionally, it was reported that the non-diarrheic piglets were found to have a considerably higher abundance of intestinal Prevotella than diarrheic piglets. Prevotellacea UCG-003 was the key bacterium in the non-diarrheic microbiota of piglets, according to co-correlation network analysis [23]. Ngo et al. (2021) used a new probiotic (B. amyloliquefaciens H57) in high concentrate feed pellets that reduces volatile fatty acid production and prevents flavor in pellet feed. That facilitates higher feed intake in ruminant animals [25]. In recent studies on anaerobic fungi, it was demonstrated that it contributes essentially to ruminal fiber utilization by degrading plant cell walls in two ways, i.e., enzymatically and mechanically [26][27]. Remarkably, ongoing exploration showed the affinity of fungal CAZymes for stubborn fiber, which might clarify the specific use of anaerobic fungi when lower quality forages were fed to ruminants. Therefore, this can also be used as a potential probiotic in ruminant nutrition [28]. Studies on the utilization of B. subtilis as a spore-shaping probiotic bacterium in livestock nutrition have shown no unsafe impacts and have exhibited the viability of its utilization as a probiotic, mostly because of its demonstrated AM, mitigating cell reinforcement and exhibiting enzymatic, and immunomodulatory action [29]. A study by Cai et al. (2021) enumerated that S. cerevisiae and C. butyricum and their blend enhanced rumen conditions by expanding the pH and diminishing oxidation and upgraded rumen maturation capacities by expanding absorbability of supplements and further developing VFA production; from that point on, further enhancements in production growth of heat-stressed goats were observed [30]. The Debaryomyces hansenii is also gaining attraction as a new potential probiotic for both terrestrial and aquatic animals. The oral delivery of D. Hansenii has been linked to probiotic features, such as immunostimulatory effects, gut microbiota regulation, increased cell proliferation, differentiation, and improved digestive function. Its bioactive molecules have been identified and linked to its immunomodulatory effect, including cell wall components and polyamines [31]. Therefore, there are many potential probiotic microbes that are still to be discovered, which might play an evolutionary role in livestock production.
3. Modes of Action of Livestock Probiotics
There are numerous proposed modes of action of livestock probiotics [32][33][34][35][36][37][38][39][40]. However, the major mechanisms of action proposed for probiotics are considered in the following segments (summarized in Figure 2).
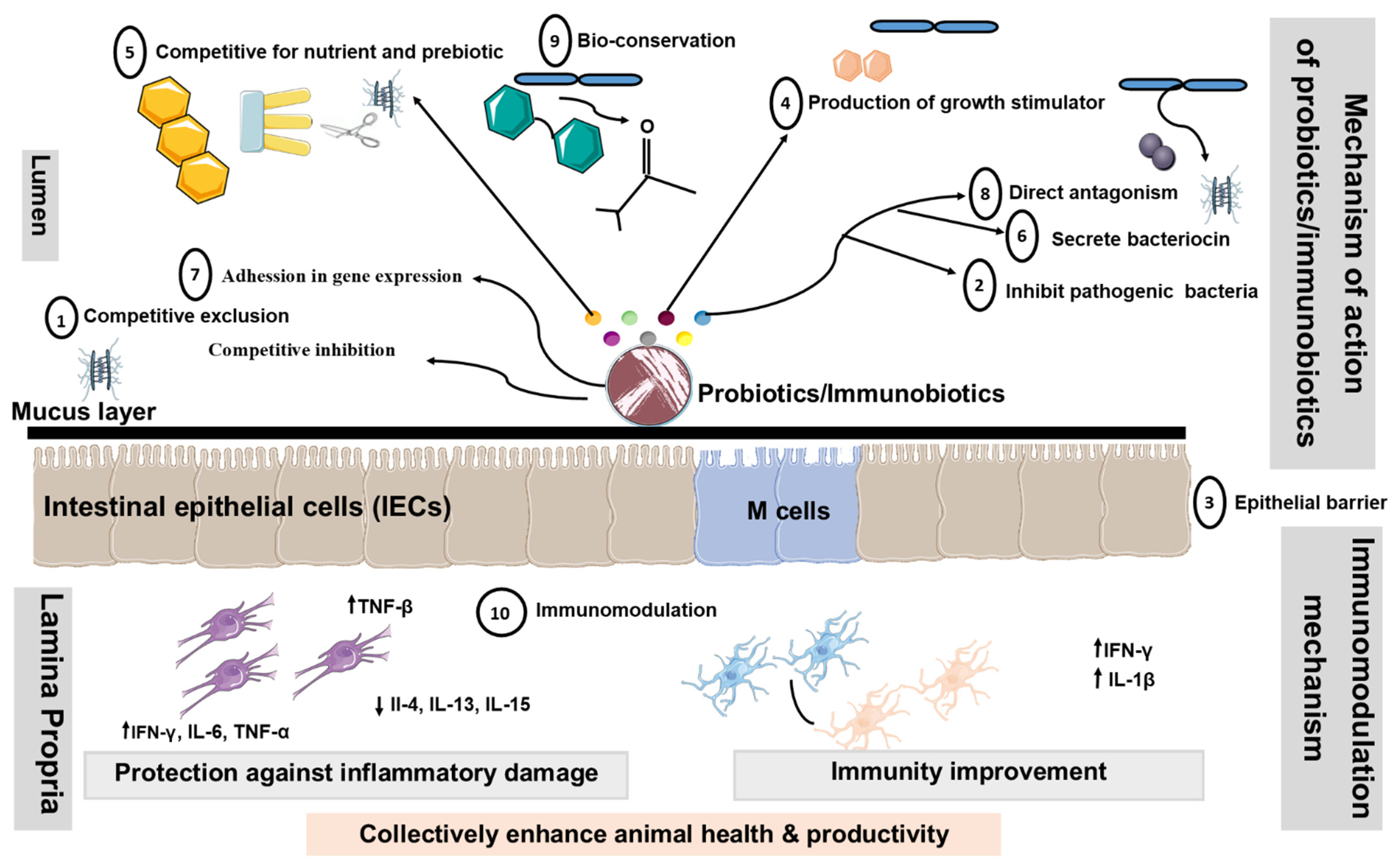
Figure 2. Proposed modes of action of livestock probiotics. Schematic diagram illustrating potential mechanisms, whereby oral administration of probiotics might promote beneficial effects by changing the composition of intestinal microbiota, altering intestinal barrier function, bile salts, and production of Th1 cytokines. Additionally, probiotics containing LAB may down-regulate the expression of pro-inflammatory cytokines and chemokines. Decrease in the translocation of bacteria may occur as a result of the ability of probiotics to tighten the mucosal barrier. Probiotics disallow colonization by pathogenic bacteria through competition for nutrients, immune system up-regulation, and production of antitoxins. These mechanisms include ① Competitive exclusion for binding sites, ② Adhesion to the GIT,③ Enhancement of the epithelial barrier, ④ Increase in digestion and absorption of nutrients, ⑤ Competing with pathogenic bacteria for nutrients in the gut, ⑥ Production of AM substances, ⑦ Alteration in gene expression in pathogenic microorganisms, ⑧ Bacterial antagonism, ⑨ Bioconversion and ⑩ Immunomodulation. Abbreviations: ↑, increased; ↓, decreased; Th1, Type 1 T helper; Th2, Type 2 T helper; IEC, intestinal epithelial cells; DC: dendritic cell.
① Modification of the microbial population of the GIT: Probiotics might boost the population of beneficial microbes, such as Lactobacillus and Bifidobacterium, which subsequently restrict the growth of harmful bacteria by creating inhibitory chemicals and by competing for binding sites [41][42]. ② Adhesion to the GIT wall to prevent colonization by pathogenic microorganisms: The majority of enteric pathogens might colonize the intestinal epithelium and cause disease as a result [43]. As a result, Lactobacillus can adhere to the gut epithelium and compete with pathogens for adhesion receptors, such as glycoconjugates [44]. The Lactobacillus and Bifidobacterium have hydrophobic surface layer proteins that assist the bacteria non-specifically by adhering to the animal cell surface [45]. ③ Enhancement of the Epithelial Barrier: The experimental studies in model animal have shown that probiotics P. acidilactici improve intestinal barrier function by reducing the permeability of the intestinal epithelium translocation of enterotoxigenic E. coli to mesenteric lymph nodes in post-weaning piglets as compared to the control group after ETEC challenge
④ Increase in digestion and absorption of nutrients: In this case, the spore-forming bacteria enhance the production of extracellular enzymes, which facilitate nutrient digestion [48][49]. ⑤ Competing with pathogenic bacteria for nutrients in the gut: Probiotic bacteria might compete with pathogenic bacteria for nutrients and absorption sites by rapidly utilizing energy sources, potentially shortening the log phase of bacterial development [42]. ⑥ Production of antimicrobial substances: Several probiotic bacteria, particularly those that produce lactic and acetic acids, have the ability to suppress harmful microorganisms [50][51]. ⑦ Alteration in gene expression in pathogenic microorganisms: Probiotics might influence pathogenic bacteria’s quorum sensing, hence altering their pathogenicity. Fermentation products from L. acidophilus La-5 significantly suppressed the extracellular production of a chemical signal (autoinducer-2) by human enterohaemorrhagic E. coli serotype O157:H7, leading to inhibition of the virulent gene (LEE—locus of enterocyte effacement) expression in vitro [52]. ⑧ Bacterial antagonism: Probiotic microorganisms, once established in the gut, may produce organic acids, hydrogen peroxide, lactoferrin, and bacteriocin, which may exhibit either bactericidal or bacteriostatic properties [53].⑨ Bactericidal activity/Bioconversion: Lactobacillus convert lactose to lactic acid, lowering the pH to a point where pathogenic bacteria cannot survive. Furthermore, living yeasts compete with lactic acid-producing bacteria to digest sugars obtained from starch breakdown, thereby stabilizing rumen pH and minimizing the danger of acidosis [54][55][56]. ⑩ Immunomodulation: It is shown that probiotic LAB with immunoregulatory functions can beneficially modulate the immune response in the gut by modulating the functions of PIE cells [12][57][58]. In addition, probiotic LAB have proven to be capable of acting as immune modulators by enhancing macrophage activity [57], increasing local antibody levels, inducing the production of anti-inflammation cytokines (interleukin (IL)- 10, interferon (IFN)-γ, β, IL-1β, TGF-β), reducing IL-4, IL-6, IL-8, MCP-1, and activating killer cells [11][59][57].
Immunomodulation properties appear to be strain dependent, which means that dissimilar probiotics might have parallel mechanisms of action, whereas a single strain may have multiple mechanisms of action. Quite a lot of probiotic strains, for example, have comparable impact on the microbial community of gastrointestinal tract, although the mechanisms of action of certain probiotics are mostly unknown. The exact mode of action of probiotics is not well understood in the majority of studies on their impact on performance. Therefore, the mechanisms must be explored on a case-by-case basis because closely interrelated probiotics appear to have diverse ways of action. Probiotic effects are a result of the interaction between the host and the probiotic microorganism. As a result, more research into the host–microbe interaction could shed light on the probiotic mode of action. Rapid improvements in molecular techniques and genome sequencing for microbial ecology research will substantially aid our understanding of probiotic mechanisms of action.
References
- Komarek, A.M.; Dunston, S.; Enahoro, D.; Godfray, H.C.J.; Herrero, M.; Mason-D’Croz, D.; Rich, K.M.; Scarborough, P.; Springmann, M.; Sulser, T.B.; et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob. Environ. Change 2021, 70, 102343.
- Hassan, M.M.; El Zowalaty, M.E.; Lundkvist, Å.; Järhult, J.D.; Khan Nayem, M.R.; Tanzin, A.Z.; Badsha, M.R.; Khan, S.A.; Ashour, H.M. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021, 111, 141–150.
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Babu Rajendran, N.; Voss, A. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin. Microbiol. Infect. 2018, 24, 577–590.
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918.
- Cuevas-González, P.F.; Peredo-Lovillo, A.; Castro-López, C.; Vallejo-Cordoba, B.; González-Córdova, A.F.; García, H.S.; Hernández-Mendoza, A. Food-grade lactic acid bacteria and probiotics as a potential protective tool against erythrotoxic dietary xenobiotics. Trends Food Sci. Technol. 2021, 116, 1041–1055.
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019, 223, 84–96.
- Gibson, M.K.; Crofts, T.S.; Dantas, G. Antibiotics and the developing infant gut microbiota and resistome. Curr. Opin. Microbiol. 2015, 27, 51–56.
- Tavoukjian, V. Faecalmicrobiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188.
- Andremont, A.; Cervesi, J.; Bandinelli, P.-A.; Vitry, F.; de Gunzburg, J. Spare and repair the gut microbiota from antibiotic-induced dysbiosis: State-of-the-art. Drug Discov. Today 2021, 26, 2159–2163.
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.Z.; Saito, T.; Kitazawa, H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 2013, 8, e59259.
- Suda, Y.; Villena, J.; Takahashi, Y.; Hosoya, S.; Tomosada, Y.; Tsukida, K.; Shimazu, T.; Aso, H.; Tohno, M.; Ishida, M.; et al. Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs. BMC Immunol. 2014, 15, 24.
- Fujie, H.; Villena, J.; Tohno, M.; Morie, K.; Shimazu, T.; Aso, H.; Suda, Y.; Shimosato, T.; Iwabuchi, N.; Xiao, J.Z.; et al. Toll-like receptor-2-activating bifidobacteria strains differentially regulate inflammatory cytokines in the porcine intestinal epithelial cell culture system: Finding new anti-inflammatory immunobiotics. FEMS Immunol. Med. Microbiol. 2011, 63, 129–139.
- Villena, J.; Salva, S.; Núñez, M.; Corzo, J.; Tolaba, R.; Faedda, J.; Font, G.; Alvarez, S. Probiotics for Everyone! The Novel Immunobiotic Lactobacillus rhamnosus CRL1505 and the Beginning of Social Probiotic Programs in Argentina. Int. J. Biotechnol. Wellness Ind. 2012, 189–198.
- Kumagae, N.; Villena, J.; Tomosada, Y.; Kobayashi, H.; Kanmani, P.; Aso, H.; Sasaki, T.; Yoshida, M.; Tanabe, H.; Shibata, I.; et al. Evaluation of the Immunoregulatory Capacities of Feed Microbial Materials in Porcine Intestinal Immune and Epithelial Cells. Open J. Vet. Med. 2014, 4, 14.
- Kobayashi, H.; Kanmani, P.; Ishizuka, T.; Miyazaki, A.; Soma, J.; Albarracin, L.; Suda, Y.; Nochi, T.; Aso, H.; Iwabuchi, N.; et al. Development of an in vitro immunobiotic evaluation system against rotavirus infection in bovine intestinal epitheliocytes. Benef. Microbes 2017, 8, 309–321.
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79.
- Kwak, M.-J.; Tan, P.L.; Oh, J.K.; Chae, K.S.; Kim, J.; Kim, S.H.; Eun, J.-S.; Chee, S.W.; Kang, D.-K.; Kim, S.H.; et al. The effects of multispecies probiotic formulations on growth performance, hepatic metabolism, intestinal integrity and fecal microbiota in growing-finishing pigs. Anim. Feed Sci. Technol. 2021, 274, 114833.
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18(+) in pigs. Anim. Nutr./Zhongguoxu Mu Shouyixuehui 2021, 7, 326–333.
- Sobrino, O.J.; Alba, C.; Arroyo, R.; Pérez, I.; Sariego, L.; Delgado, S.; Fernández, L.; de María, J.; Fumanal, P.; Fumanal, A.; et al. Replacement of Metaphylactic Antimicrobial Therapy by Oral Administration of Ligilactobacillus salivarius MP100 in a Pig Farm. Front. Vet. Sci. 2021, 8, 666887.
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of Early-Life Fecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhoea. PLoS ONE 2017, 12, e0169851.
- Yang, Q.; Huang, X.; Zhao, S.; Sun, W.; Yan, Z.; Wang, P.; Li, S.; Huang, W.; Zhang, S.; Liu, L.; et al. Structure and Function of the Fecal Microbiota in Diarrheic Neonatal Piglets. Front. Microbiol. 2017, 8, 502.
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12.
- Sun, J.; Du, L.; Li, X.; Zhong, H.; Ding, Y.; Liu, Z.; Ge, L. Identification of the core bacteria in rectums of diarrheic and non-diarrheic piglets. Sci. Rep. 2019, 9, 18675.
- Liu, Y.; Zheng, Z.; Yu, L.; Wu, S.; Sun, L.; Wu, S.; Xu, Q.; Cai, S.; Qin, N.; Bao, W. Examination of the temporal and spatial dynamics of the gut microbiome in newborn piglets reveals distinct microbial communities in six intestinal segments. Sci. Rep. 2019, 9, 3453.
- Ngo, T.T.; Bang, N.N.; Dart, P.; Callaghan, M.; Klieve, A.; Hayes, B.; McNeill, D. Feed Preference Response of Weaner Bull Calves to Bacillus amyloliquefaciens H57 Probiotic and Associated Volatile Organic Compounds in High Concentrate Feed Pellets. Animals 2021, 11, 51.
- Gruninger, R.J.; Puniya, A.K.; Callaghan, T.M.; Edwards, J.E.; Youssef, N.; Dagar, S.S.; Fliegerova, K.; Griffith, G.W.; Forster, R.; Tsang, A.; et al. Anaerobic fungi (phylum Neocallimastigomycota): Advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol. Ecol. 2014, 90, 1–17.
- Hess, M.; Paul, S.S.; Puniya, A.K.; van der Giezen, M.; Shaw, C.; Edwards, J.E.; Fliegerová, K. Anaerobic Fungi: Past, Present, and Future. Front. Microbiol. 2020, 11, 584893.
- Hagen, L.H.; Brooke, C.G.; Shaw, C.A.; Norbeck, A.D.; Piao, H.; Arntzen, M.Ø.; Olson, H.M.; Copeland, A.; Isern, N.; Shukla, A.; et al. Proteome specialization of anaerobic fungi during ruminal degradation of recalcitrant plant fiber. ISME J. 2021, 15, 421–434.
- Ruiz Sella, S.R.B.; Bueno, T.; de Oliveira, A.A.B.; Karp, S.G.; Soccol, C.R. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369.
- Cai, L.; Yu, J.; Hartanto, R.; Qi, D. Dietary Supplementation with Saccharomyces cerevisiae, Clostridium butyricum and Their Combination Ameliorate Rumen Fermentation and Growth Performance of Heat-Stressed Goats. Animals 2021, 11, 2116.
- Angulo, M.; Reyes-Becerril, M.; Medina-Córdova, N.; Tovar-Ramírez, D.; Angulo, C. Probiotic and nutritional effects of Debaryomyces hansenii on animals. Appl. Microbiol. Biotechnol. 2020, 104, 7689–7699.
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563.
- Saleem, M.; Malik, S.; Mehwish, H.M.; Ali, M.W.; Hussain, N.; Khurshid, M.; Rajoka, M.S.R.; Chen, Y. Isolation and functional characterization of exopolysaccharide produced by Lactobacillus plantarum S123 isolated from traditional Chinese cheese. Arch. Microbiol. 2021, 203, 3061–3070.
- Riaz Rajoka, M.S.; Mehwish, H.M.; Xiong, Y.; Song, X.; Hussain, N.; Zhu, Q.; He, Z. Gut microbiota targeted nanomedicine for cancer therapy: Challenges and future considerations. Trends Food Sci. Technol. 2021, 107, 240–251.
- Riaz Rajoka, M.S.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48.
- Riaz Rajoka, M.S.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and antioxidant activity of exopolysaccharide mediated silver nanoparticle synthesized by Lactobacillus brevis isolated from Chinese koumiss. Colloids Surf. B Biointerfaces 2020, 186, 110734.
- Riaz Rajoka, M.S.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588.
- Riaz Rajoka, M.S.; Zhao, H.; Mehwish, H.M.; Li, N.; Lu, Y.; Lian, Z.; Shao, D.; Jin, M.; Li, Q.; Zhao, L.; et al. Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res. Int. 2019, 123, 286–297.
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715.
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1132–1142.
- Mountzouris, K.C.; Balaskas, C.; Xanthakos, I.; Tzivinikou, A.; Fegeros, K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009, 50, 467–478.
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed Sci. Technol. 2015, 200, 86–92.
- Walker, W.A. Role of nutrients and bacterial colonization in the development of intestinal host defense. J. Pediatr. Gastroenterol. Nutr. 2000, 30 (Suppl. 2), S2–S7.
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr./Zhongguoxu Mu Shouyixuehui 2017, 3, 1–6.
- Johnson-Henry, K.C.; Hagen, K.E.; Gordonpour, M.; Tompkins, T.A.; Sherman, P.M. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell. Microbiol. 2007, 9, 356–367.
- Lessard, M.; Dupuis, M.; Gagnon, N.; Nadeau, E.; Matte, J.; Goulet, J.; Fairbrother, J. Administration of Pediococcus Acidilactici or Saccharomyces CerevisiaeBoulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia Coli challenge. J. Anim. Sci. 2008, 87, 922–934.
- Sato, N.; Garcia-Castillo, V.; Yuzawa, M.; Islam, M.A.; Albarracin, L.; Tomokiyo, M.; Ikeda-Ohtsubo, W.; Garcia-Cancino, A.; Takahashi, H.; Villena, J.; et al. Immunobiotic Lactobacillus jensenii TL2937 Alleviates Dextran Sodium Sulfate-Induced Colitis by Differentially Modulating the Transcriptomic Response of Intestinal Epithelial Cells. Front. Immunol. 2020, 11, 2174.
- Gangadharan, D.; Sivaramakrishnan, S.; Nampoothiri, K.M.; Sukumaran, R.K.; Pandey, A. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour. Technol. 2008, 99, 4597–4602.
- Lee, Y.J.; Kim, B.K.; Lee, B.H.; Jo, K.I.; Lee, N.K.; Chung, C.H.; Lee, Y.C.; Lee, J.W. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 2008, 99, 378–386.
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.H.; Liévin-Le Moal, V.; Servin, A.L. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013.
- Daşkıran, M.; Önol, A.G.; Cengiz, Ö.; Ünsal, H.; Türkyılmaz, S.; Tatlı, O.; Sevim, Ö. Influence of dietary probiotic inclusion on growth performance, blood parameters, and intestinal microflora of male broiler chickens exposed to posthatch holding time. J. Appl. Poult. Res. 2012, 21, 612–622.
- Higgins, D.A.; Pomianek, M.E.; Kraml, C.M.; Taylor, R.K.; Semmelhack, M.F.; Bassler, B.L. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 2007, 450, 883–886.
- Pringsulaka, O.; Rueangyotchanthana, K.; Suwannasai, N.; Watanapokasin, R.; Amnueysit, P.; Sunthornthummas, S.; Sukkhum, S.; Sarawaneeyaruk, S.; Rangsiruji, A. In vitro screening of lactic acid bacteria for multi-strain probiotics. Livest. Sci. 2015, 174, 66–73.
- Oh, B.-T.; Jeong, S.-Y.; Velmurugan, P.; Park, J.-H.; Jeong, D.-Y. Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J. Biosci. Bioeng. 2017, 124, 542–550.
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chem. 2021, 342, 128328.
- García, C.; Rendueles, M.; Díaz, M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220.
- Shimazu, T.; Villena, J.; Tohno, M.; Fujie, H.; Hosoya, S.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; Saito, T.; et al. Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway. Infect. Immun. 2012, 80, 276–288.
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 2014, 14, 126.
- Suda, Y.; Sasaki, N.; Kagawa, K.; Elean, M.; Zhou, B.; Tomokiyo, M.; Islam, M.A.; Rajoka, M.S.R.; Kober, A.K.M.H.; Shimazu, T.; et al. Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs. Microorganisms 2021, 9, 921.
More
Information
Subjects:
Agriculture, Dairy & Animal Science; Immunology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
954
Revisions:
3 times
(View History)
Update Date:
11 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




