Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jakub Kurek | + 3833 word(s) | 3833 | 2022-02-28 09:32:00 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3833 | 2022-03-07 10:44:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kurek, J. L-Arginine in Disturbed Carbohydrate Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/20279 (accessed on 11 January 2026).
Kurek J. L-Arginine in Disturbed Carbohydrate Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/20279. Accessed January 11, 2026.
Kurek, Jakub. "L-Arginine in Disturbed Carbohydrate Treatment" Encyclopedia, https://encyclopedia.pub/entry/20279 (accessed January 11, 2026).
Kurek, J. (2022, March 07). L-Arginine in Disturbed Carbohydrate Treatment. In Encyclopedia. https://encyclopedia.pub/entry/20279
Kurek, Jakub. "L-Arginine in Disturbed Carbohydrate Treatment." Encyclopedia. Web. 07 March, 2022.
Copy Citation
L-arginine, an endogenous amino acid, is a safe substance that can be found in food. The compound is involved in synthesis of various products responsible for regulatory functions in the body. Particularly noteworthy is, among others, nitric oxide, a signaling molecule regulating carbohydrate and lipid metabolism. The increasing experimental and clinical data indicate that L-arginine supplementation may be helpful in managing disturbed metabolism in obesity, regulate arterial blood pressure or alleviate type 2 diabetes symptoms, but the mechanisms underlying these effects have not been sufficiently elucidated.
L-arginine
nitric oxide
carbohydrate metabolism
lipid metabolism
1. Introduction
L-arginine (2-Amino-5-guanidinovaleric acid-Arg) is an endogenous amino acid, which is mainly formed in the urea cycle. It is involved in synthesis of proteins, urea, creatine, prolamines (purtescin, spermine, spermidine), proline and nitric oxide (NO). The end products of arginine metabolism are NO, glutamate and prolamins that have various regulatory functions in the body [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15].
NO is a signaling molecule, which at physiological levels stimulates glucose uptake as well as glucose and fatty acid oxidation in skeletal muscle, heart, liver and adipose tissue; inhibit the synthesis of glucose, glycogen, and fat in target tissues (e.g., liver and adipose); and enhance lipolysis in adipocytes. Thus, an inhibition of NO synthesis causes hyperlipidemia and fat accretion in rats, whereas dietary arginine supplementation reduces fat mass in diabetic fatty rats [16].
The putative underlying mechanisms on NO action were extensively presented in a number of previous publications of this topic [8][11][16][17][18][19][20][21][22][23][24][25], thus will not be repeated in detail in this entry.
In short, L-arginine is a safe substance that can be found in food [20]. An adult human consumes approximately 5.4 g of arginine per day [5]. Doses of 3–8 g/d appear to be safe and not to cause acute pharmacologic effects in humans [20].
One of the causes for carbohydrate and lipid metabolism disorders in the organism may be deficiency or excess of NO. Therefore, the use of L-arginine in the prevention and treatment of lipid and carbohydrate metabolism disorders should be investigated.
Results of recent studies indicates that L-arginine supplementation may reduce obesity, improve arterial blood pressure, mitigate oxidation and regulate endothelial dysfunction that can be helpful in managing various metabolic disorders, including type 2 diabetes mellitus (T2DM). The mechanisms of L-arginine regulatory effects may result from its contribution in stimulating lipolysis, promoting the endocrine system functions, ameliorating insulin sensitivity, restoring glucose homeostasis and fetal programming. Some in vivo studies reported that L-arginine may restore insulin sensitivity and thus mitigate T2DM [26].
2. Transport and Absorption of L-Arginine
L-arginine is provided with food in the form of compounds with other amino acids-proteins. Absorption occurs mainly in the ileum and jejunum. Due to the high arginase activity in the small intestine, absorption of arginine in the portal vein occurs in about 60%. The remaining 40% is degraded. It is assumed that ultimately about 50% of dietary arginine enters the circulatory system [8]. Additionally, 5–15% of L-arginine in the blood comes from endogenous synthesis (from L-citrulline and L-ornithine) [5][8][11]. It can also be provided, to a lesser extent, by intracellular protein degradation [11].
Arginine reaches its maximum concentration in the blood approximately 2 h after oral administration. With an oral administration of 6 g, the biological half-life is 1.5–2 h [10].
Arginine is transported from the blood into the cells by specific protein transporters [9]. One such transporter is the CAT-1 protein, often found on the membrane surface together with endothelial nitric oxide synthase (eNOS). Together they form a complex responsible for the delivery of L-arginine to eNOS. This is a mechanism for direct targeting of extracellular L-arginine for NO synthesis, thus optimising its production in endothelial cells [12][13][14]. However, most of the L-arginine used to produce NO comes from the efficient recycling of L-citrulline (derived from L-arginine) back to L-arginine, which is dependent on the capacity of endothelial cells [27]. On the other hand, some studies indicate a higher importance of extracellular arginine in the production of NO [14]. A simplified pathway showing the conversion of L-arginine to NO is shown in Figure 1.
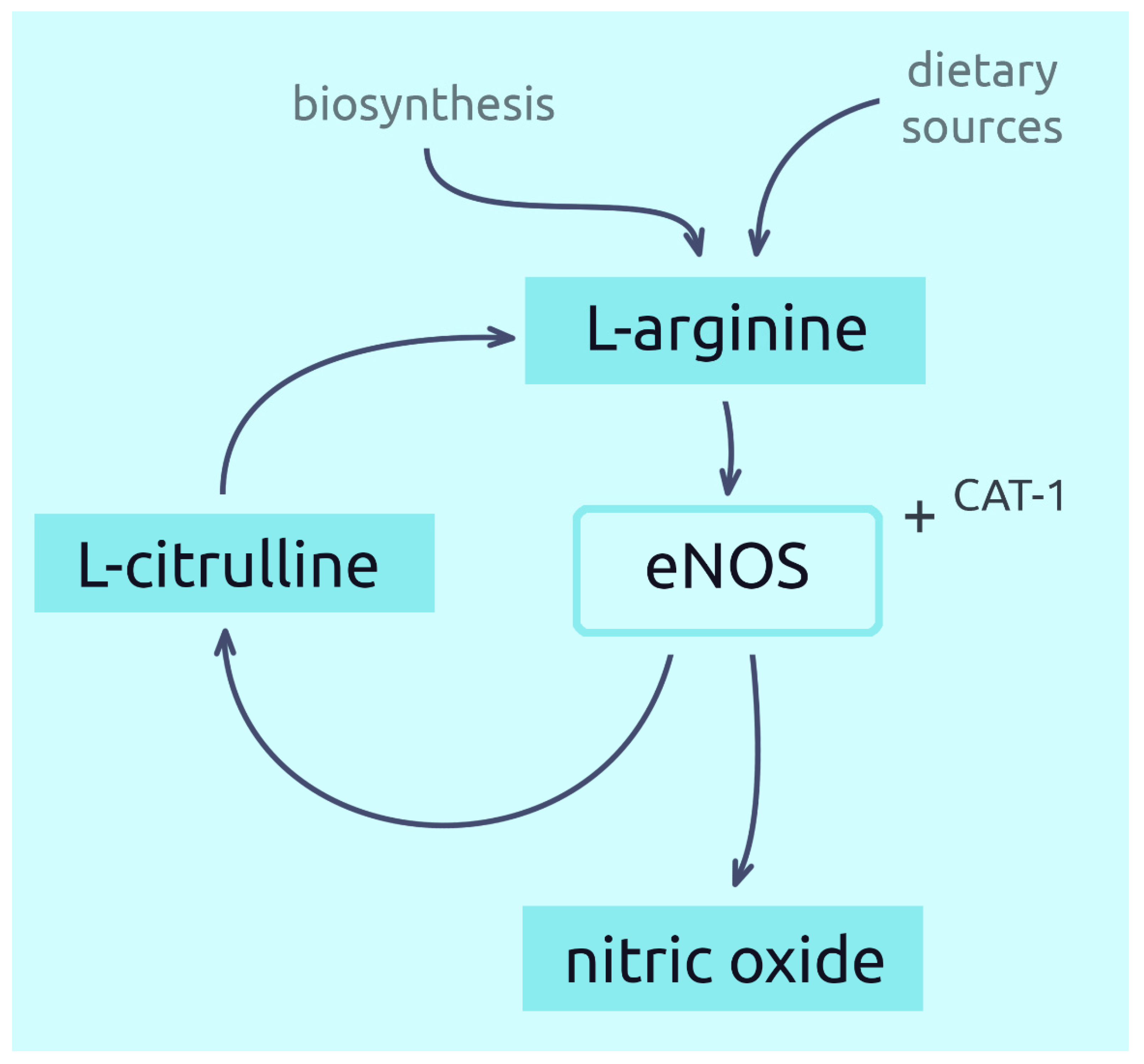
Figure 1. Synthesis of nitric oxide from L-arginine.
3. Current Uses and Potential Properties of L-Arginine
L-arginine is currently used in case of liver dysfunctions (especially related to urea cycle abnormalities), ammonia poisoning, asthenic disorders and malnutrition, as well as a supportive amino acid for athletes [28].
It can also be administered intravenously to boost growth hormone (in case of deficiency), used in diagnostic detection of growth hormone deficiency [29][30][31][32] or as an acidifier in extreme metabolic alkalosis [29].
Potentially, L-arginine may find use in various types of hypertension, ischemic heart disease, heart failure, atherosclerosis (especially of the lower limbs), hypercholesterolemia, glaucoma, Raynaud’s phenomenon, chronic kidney failure, diabetes and prevention of cardiovascular disease, including stroke [20][33][34]. Despite its positive effect on many cardiovascular diseases, L-arginine supplementation may increase mortality after myocardial infarction. This effect potentially results in an increase in the production of NO, peroxynitrite, levels of NOS inhibitors and reactive oxygen species (due to impaired eNOS activity). For this reason, it is not recommended for people who has a myocardial infarction and should be used with caution in the elderly [35].
4. Potential of L-Arginine in the Treatment of Carbohydrate Metabolism Disorders
Despite conducting numerous studies, the exact effect and mechanism of action of L-arginine on carbohydrate metabolism disorders and its complications still cannot be determined. Studies often show various, contradictory results. However, the ones that can be spotted in the research most commonly and that show the greatest therapeutic potential of L-arginine are the improvement of insulin sensitivity, reduction of inflammation and oxidative stress, an increase of NO levels and vasodilation of vessels. In long-term use, L-arginine may also improve glucose tolerance and even reduce the risk of diabetes. The results of studies on the use of L-arginine in disorders related to carbohydrate metabolism are presented in Table 1.
Table 1. Effects of L-arginine in the treatment of carbohydrate metabolism disorders.
| Cell Testing | ||||||
| Study (Year) | Cell Line/Model | Dose(s) of L-Arginine Tested |
Control Culture | Outcome | ||
| Adeghate et al. (2001) [36] | pancreas fragments of diabetic rats |
100 mM | + | L-arginine stimulates insulin secretion | ||
| Pi et al. (2012) [37] | pancreatic islets of Gprc6a−/− mice | 10 mM | + | L-arginine stimulates insulin secretion in β-cells through GPRC6A activation of cAMP pathways | ||
| Smajilovic et al. (2013) [38] | pancreatic islets of Gprc6a−/− mice | 20 mM | + | L-arginine induces insulin secretion, but GPRC6A is not involved in the process | ||
| Krause et al. (2011) [39] | BRIN-BD11 | 0.1, 0.25, 1.15 mM | + | L-arginine induces insulin secretion, contributes to glutathione synthesis and has a protective effects in the presence of proinflammatory cytokines | ||
| Tsugawa et al. (2019) [40] | Hep G2 | 1, 3.3, 10 mM | + | L-arginine increase IGF-1 level by stimulating of growth hormone secretion | ||
| Cho et al. (2020) [41] | NIT-1 + HEK293FT | 0.1, 0.2, 0.6, 1, 2 mM | + | L-arginine induces insulin secretion due to UGGT1 regulatory functions | ||
| Animal Testing | ||||||
| Study, Year | Duration of Experiment |
Dose(s) of L-Arginine Tested |
Control Group |
Number of Animals per Group |
Animal Model |
Outcome |
| Smajilovic et al. (2013) [38] | 1 min | 0.05 g/kg bw intravenously + 1 g/kg bw orally |
+ | 6–10 | Gprc6a−/− mice | Increase in insulin secretion after intravenous injection and oral administration of L-arginine |
| Tsugawa et al. (2019) [40] | 120 min | 3 mg/kg bw orally | + | 4 | C57BL/6J mice | L-arginine induces secretion of growth hormone and IGF-1 |
| Cho et al. (2020) [41] | 120 min | 0.75, 1.5, 3 mg/g intraperitoneally | + | - | β cell-specific UGGT1-transgenic mice |
UGGT1 mediated proinsulin management regulates insulin secretion |
| Kohli et al. (2004) [42] | 2 weeks | 0.64% in diet + 1.25% in water | + | 8 | Sprague-Dawley rats | L-arginine stimulates endothelial NO synthesis by increasing BH4 concentration, increased insulin concentration in the blood and reduced blood glucose level in diabetic rats |
| Fu et al. (2005) [43] | 10 weeks | 1.44% in diet + 1.25% in water | + | 6 | Zucker diabetic fatty rats | L-arginine increases NO synthesis, lower glucose level and reduce body weight in obese and type 2 diabetic rats |
| Clemmensen et al. (2013) [44] | 15/120 min | 1 g/kg bw orally | + | 7–17 | C57BL/6 mice + Glp1r−/− mice | L-arginine increases GLP-1 and insulin levels and improves glucose clearance in obese mice; effects depends on GLP-1R-signaling |
| El-Missiry et al. (2004) [45] | 1 week | 100 mg/kg bw intragastrically | + | 6–8 | Wistar rats | L-arginine lowers serum glucose and oxidative stress in diabetic rats |
| Ortiz et al. (2013) [46] | 4 days | 622 mg/kg bw/day in water |
+ | 5 | Wistar rats | L-arginine ameliorates oxidative stress and the decrease in NO production in diabetic rats |
| Pai et al. (2010) [47] | 8 weeks | 1.5 g/kg bw/day orally | + | 6–13 | Wistar rats | L-arginine has no effect on plasma glucose levels, but decreases advanced glycation endproducts in diabetic rats |
| Human Research | ||||||
| Study, Year | Duration of Experiment |
Dose(s) of L-Arginine Tested |
Control Group |
Number of Subjects per Group |
Outcome | |
| Wascher et al. (1997) [48] | - | 0.52 mg/kg−1 bw/ min−1 (concomitant infusion) |
+ | 7–9 | L-arginine improves insulin sensitivity and restores vasodilatation (insulin-mediated) in obese and non-insulin-dependent diabetic patients; no effects was observed on insulin or IGF-1 levels | |
| Piatti et al. (2001) [49] | 3 months (1 month of intervention) | 3 × 3 g/day orally | + | 12–40 | L-arginine normalizes cGMP levels, improves glucose disposal and systolic blood pressure; the treatment attenuates insulin resistance in type 2 diabetic patients | |
| Lucotti et al. (2006) [50] | 3 weeks | 8.3 g/day orally | + | 16–17 | L-arginine positively affects glucose metabolism and insulin sensitivity, improves endothelial function, oxidative stress, and adipokine release in obese type 2 diabetic patients | |
| Lucotti et al. (2009) [51] | 6 months | 6.4 g/day orally | + | 32 | L-arginine regulates endothelial dysfunction, improves insulin sensitivity and reduces inflammation | |
| Bogdański et al. (2012) [52] | 3 months | 3 × 9 g/day orally | + | 20 | L-arginine decreases insulin level and improves insulin sensitivity; TNF-alpha plays role in the pathogenesis of insulin resistance in patients with obesity | |
| Jabłecka et al. (2012) [53] | 2 months | 3 × 2 g/day orally | + | 12–38 | L-arginine does not affect fasting glucose and HbA1 level in diabetic patients with atherosclerotic peripheral arterial disease, but increases NO and TAS levels | |
| Bogdanski et al. (2013) [54] | 6 months | 3 × 9 g/day orally | + | 44 | L-arginine decreases plasminogen activator type 1, increases NO and TAS levels, and improves insulin sensitivity in obese patients | |
| Suliburska et al. (2014) [55] | 6 months | 3 × 9 g/day orally | + | 44 | L-arginine affects zinc serum concentrations in obese patients; positive correlation between the change in zinc and insulin sensitivity improvement was observed | |
| Monti et al. (2013) [56] | 6 weeks (2 weeks of intervention) | 6.6 g/day orally | cross-over study | 7–8/15 | L-arginine improves glucose metabolism, insulin secretion and insulin sensitivity; it enhances endothelial function in patients with impaired glucose tolerance and metabolic syndrome | |
| Monti et al. (2012) [57] | 18 months + 12-month follow-up period | 6.4 g/day orally | + | 72 | L-arginine improves β-cell function and insulin sensitivity, and increase probability to become normal glucose tolerant, but does not reduce the incidence of diabetes in patients with impaired glucose tolerance and metabolic syndrome | |
| Monti et al. (2018) [58] | 18 months + 90-month follow-up | 6.4 g/day orally | + | 45–47 | L-arginine delays the development of T2DM; the effect could be related to reduction in oxidative stress | |
4.1. Cell Testing
One of the most important organs of carbohydrate metabolism is the pancreas. For this reason, most research relies on pancreatic cell lines.
Adeghate et al. (2001) studied the effect of L-arginine on insulin secretion by the pan-creas. Pancreas sections of type 2 diabetic and healthy rats were incubated in solutions with L-arginine. Insulin secretion in the pancreas of diseased rats was significantly stimulated by L-arginine [36]. G protein-coupled receptor family C group 6 member A (GPRC6A) is a membrane androgen receptor with a high importance for energy metabolism, hormone production and glucose homeostasis. Pi et al. (2012) demonstrated that L-arginine regulates insulin secretion through expression of GPRC6A in isolated mouse pancreatic islets. Gprc6a−/− mice from which cells were harvested had metabolic syndrome including, among others, obesity and glucose intolerance. In addition, the expression of insulin was significantly lower in islets derived from Gprc6a−/− mice compared with normal mice. This response was selective for insulin, as glucagon expression was not impaired in Gprc6a−/− mice. GPRC6A is expressed in βcells in the pancreas, and one of its substrates is L-arginine. L-arginine provided to mouse cells with GPRC6A stimulated cAMP accumulation ex vivo through GPRC6A dependent mechanisms. L-arginine in the presence of both low and high glucose medium stimulated insulin secretion in islets of wild-type mice (with GPRC6A). L-arginine-stimulated insulin secretion rate was significantly lower in islets isolated from Gprc6a−/− mice. This suggests that GPRC6A stimulated by L-arginine can regulate insulin secretion by βcells in the pancreas [37]. In contrast, Smajilovic et al. (2013) showed that GPRC6A is expressed in islets of Langerhans, but activation of this receptor by L-arginine does not stimulate insulin secretion. The Gprc6a−/− mice from which the cells were collected did not show any metabolic abnormalities, compared to wild-type mice. The isolated cells, after incubation with L-arginine, did not show any changes related to insulin secretion. When glucose and L-arginine were orally administered, no changes in insulin secretion were also observed between wild-type and Gprc6a−/− mice. This research suggests that GPRC6A has no essential function in blood glucose homeostasis under normal physiological conditions [38]. The differences in the results may be explained by the use of a different method of obtaining the animal model used. More specifically, the authors of the studies described above removed other exons in the process of creating Gprc6a−/− mice. Different knockout models of the same gene may result in different phenotypes and therefore lead to different body responses to L-arginine. BRIN-BD11 are the β cells of the pancreas that are primarily responsible for secreting insulin in response to glucose. Their action can be disturbed, for example, by proinflammatory cytokines, which are one of the factors in the development of diabetes. Krause et al. (2011) conducted a study on the effects of L-arginine on using this cell line, in the absence or presence of proinflammatory cytokines mixture. The study showed that L-arginine at a concentration of 1.15 mM increased β cell survival by approximately 54% (to 96.3% cell survival) when exposed to proinflammatory cytokines. In the absence of exposure to cytokines, it increased survival by about 39% (to 100% cell survival). L-arginine also increased levels of glutathione and glutamate in cells, but decreased the ratio of glutathione disulphide to glutathione and decreased the release of glutamate from cells. It also stimulated higher glucose consumption and lactate production, particularly during cytokine exposure of cells. It partially attenuated the negative effect of cytokines on insulin secretion. L-arginine also stimulated acute insulin secretion, but this action was inhibited in the presence of proinflammatory cytokines. These results demonstrate the great importance of L-arginine in inflammatory reactions occurring, e.g., concerning T2DM [39].
The liver is another pivotal organ for the metabolism of carbohydrates. One of its functions is the secretion of the insulin-like growth factor (IGF-1). IGF-1 inhibits glucose synthesis in liver cells and by increasing glucose transporters synthesis it increases peripheral glucose uptake. It also reduces insulin secretion. Tsugawa et al. (2019) in a study on HepG2 liver cells and C57BL/6J mice showed that L-arginine-induced IGF-1 secretion occurs through a minimum of two mechanisms. The first is the induction of growth hormone secretion by L-arginine, which stimulates the translation and secretion of IGF-1. The second mechanism is a decrease in IGF-1 retention in the endoplasmic reticulum (ER), which leads to increased IGF-1 secretion [40]. In an experiment conducted on mouse NIT-1 pancreatic beta-cell line and the HEK293FT cell line it was found that proinsulin is retained in the ER by UDP-glucose: glycoprotein glucosyltransferase 1 (UGGT1) when arginine availability is limited. L- and D-arginine release proinsulin from UGGT1 through competition with proinsulin and promote proinsulin transfer from the ER into the Golgi apparatus. This ability has been demonstrated in several β-cell models. Therefore, it may be used in the precisely regulated insulin secretion induced by arginine [41].
A number of studies indicate that L-arginine may beneficially affect carbohydrate metabolism in various ways. The effect is mainly due to the properties that regulate insulin secretion, but also the release of IGF-1 and the mitigation of inflammation. The inconsistency of the results of a few studies indicates that the mechanisms responsible for the action of L-arginine require more investigation and explanation.
4.2. Animal Testing
Recently, few in vivo experiments has been done on the effects of L-arginine. This may be due to the fact that the compound is safe for administration to humans, making it available to use in clinical trials. The results of the available studies on the most important issues related to the effects of L-arginine on carbohydrate metabolism in animals are described below.
Diabetes mellitus is a disease in which a deficiency of NO and L-arginine may be observed. This was confirmed, for example, by Kohli et al. (2004). They conducted a study that showed reduced arginine levels in the blood of rats with induced diabetes. Tetrahydrobiopterin (BH4) and NO levels were also lower compared to the non-diabetic groups. Dietary L-arginine supplementation stimulated endothelial NO synthesis by increasing BH4 (essential cofactor for NO synthase) concentration. As a result of arginine supplementation, the parameters of rats with induced diabetes were similar to healthy unsupplemented rats. However, supplementation also increased the value of these parameters in healthy rats. In the case of insulin, L-arginine increased its concentration in the blood of treated rats with diabetes compared to untreated rats and in healthy supplemented rats. L-arginine supplementation reduced blood glucose levels and reduced weight loss in treated rats with diabetes compared to untreated rats [42]. Fu et al. (2005) performing a study on Zucker rats with diabetes demonstrated that a number of parameters improves after L-arginine supplementation. They found that L-arginine supplementation has great potential to increase NO synthesis and reduce body weight in case of the co-occurrence of obesity and T2DM. In addition to reducing body fat, L-arginine also decreased glucose, homocysteine, free fatty acids, dimethylarginines and leptin concentrations. L-arginine increased the expression of genes responsible for fatty acid and glucose oxidation: NO synthase-1, heme oxygenase-3, AMP-activated protein kinase, and peroxisome proliferator-activated receptor gamma coactivator-1alpha [43]. This suggests not only the hypoglycaemic effect of L-arginine, but also a positive effect on the metabolic syndrome. A hormone with pivotal role in the regulation of indices related to diabetes is glucagon-like peptide-1 (GLP-1). It affects glucose lowering by increasing insulin secretion, inhibiting glucagon secretion, reducing appetite and reducing postprandial glucose. Clemmensen et al. (2013) found that oral L-arginine supplementation acts as a stimulant of GLP-1 secretion in vivo by increasing postprandial insulin secretion and thus improving glucose tolerance. In both lean and obese mice, oral L-arginine increased plasma GLP-1 and insulin and reduced the increase in postprandial glucose. To confirm the contribution of GLP-1 receptor to these effects, L-arginine was given to Glp1r−/− knockout mice and their wild-type littermates. In this test, the effect of L-arginine on wild-type mice was the same, whereas for Glp1r−/− knockout littermates it showed no effect [44]. There were also studies testing whether the earlier supply of arginine could prevent the consequences of the destruction of pancreatic cells by alloxan. El-Missiry et al. (2004) proved that oral L-arginine supplementation for 7 days, both before and after treatment with alloxan, significantly reduced the thiobarbituric acid-reactive substances (TBARS) concentrations in liver and brain to values similar to the control group. Treated rats also had higher glutathione levels and higher superoxide dismutase and catalase activities in the liver and brain. Treatment with L-arginine for 7 days before alloxan injection proved to have an impact on the control of serum glucose levels compared with treatment with L-arginine for 7 days after alloxan injection. Using L-arginine either before or after alloxan injection showed similar effects on cholesterol levels. L-arginine administered to healthy rats, did not significantly affect any of the parameters [45]. Another study, conducted by Ortiz et al. (2013), investigated the physiology of mitochondria and their production of NO in cortex cells of rats with induced diabetes and the effect of L-arginine administration on these cells. Hyperglycaemia impaired mitochondrial function and increased mitochondrial release of free radicals, whereas NO and NOS-1 production decreased significantly. One could spot an increased level of TBARS in the brain cortex, which indicated lipid peroxidation. Treatment of rats with L-arginine did not reduce glucose levels, but significantly ameliorated the oxidative stress (primarily lower TBARS level), improved mitochondrial function and prevented a decrease in NO in diabetic rats [46]. Both of these studies clearly showed that TBARS, a byproduct of lipid peroxidation, is elevated in diabetes, and L-arginine supplementation significantly lowers it. Also, both studies showed that L-arginine supplementation after injection of alloxan did not affect hyperglycemia. L-arginine may also potentially minimize the risk of diabetic complications, such as tissue damage, by affecting not only proinflammatory substances but also their receptors. A study by Pai et al. (2010) showed that L-arginine supplementation of rats with T2DM significantly reduced liver and lung receptor of advanced glycation end products expression and inflammatory protein levels, which may consequently reduce tissue damage associated with T2DM. In contrast, L-arginine did not significantly affect cholesterol and glucose levels [47].
In all studies, L-arginine showed a reduction in inflammation. In contrast, the effects on glucose and insulin levels were different. Differences in action may result from different animal models, different doses of L-arginine, time and method of their administration (in water, feed or intragastrically). The above studies also show that when supplementation with L-arginine healthy animals, does not appear to have side effects.
4.3. Human Research
Although there are a large number of publications on clinical experiments on the effects of L-arginine, it is still not sufficient to clearly recommend or reject L-arginine as a carbohydrate regulating supplement.
Apart from NO deficiency in the course of diseases, there may be disturbed transport of L-arginine, despite its adequate amount in the blood. Assumpção et al. (2010) showed that subjects with both obesity and metabolic syndrome have impaired L-arginine transport, despite normal plasma concentrations. Diminished L-arginine transport negatively affected insulin resistance and caused hyperinsulinaemia, additionally decreasing high-density lipoprotein cholesterol (HDL-C) and increasing triglycerides (TG) level. On the other hand, it did not affect NO production and platelet aggregation [59]. Rajapakse et al. (2013) investigated the effect of insulin on L-arginine transport in the body. Patients with T2DM and healthy controls received an infusion of radiolabelled L-arginine, followed by insulin, at a rate that did not affect blood glucose. In the control group, L-arginine led to a progressive increase in blood flow and a significant increase in NO precursor. After insulin administration, L-arginine clearance increased only in healthy subjects. This suggests that insulin resistance may significantly contribute to the onset and development of cardiovascular disease in individuals with T2DM through abnormal insulin-mediated regulation of L-arginine transport [60]. In addition to regulating insulin secretion, an appropriate level of tissue insulin sensitivity is also important. It allows the body to react appropriately to insulin and can prevent insulin resistance from turning into diabetes and slow the progression of diabetes. The study by Piatti et al. (2001) was the first to present that an increase in NO availability, due to L-arginine administration, can result in increased insulin sensitivity, even if it does not reach a normal level. L-arginine treatment improved glucose utilisation in the clamp technique by 34%, as well as endogenous glucose production by 29%. L-arginine administration in patients with T2DM significantly improved peripheral and hepatic insulin sensitivity but did not normalise it completely [49].
All studies consistently showed that L-arginine supplementation increases insulin sensitivity. It is possible that, in the case of impaired transport of L-arginine, which coexists with insulin resistance, its increased consumption allows it to be transported to its destination through as yet unknown mechanisms, which reduces insulin resistance. The well-proven antioxidant effect of L-arginine is also possible here. However, conflicting results were obtained as to the effects on glucose and insulin levels. They could be influenced mainly by different doses of L-arginine and different types of diseases and their severity in patients.
References
- Blantz, R.C.; Satriano, J.; Gabbai, F.; Kelly, C. Biological Effects of Arginine Metabolites: Effects of Arginine Metabolites. Acta Physiol. Scand. 2000, 168, 21–25.
- Flynn, N.E.; Meininger, C.J.; Haynes, T.E.; Wu, G. The Metabolic Basis of Arginine Nutrition and Pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427–438.
- Morris, S.M. Regulation of Enzymes of the Urea Cycle and Arginine Metabolism. Annu. Rev. Nutr. 2002, 22, 87–105.
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D.I. Arginine. Biomed. Pharmacother. 2002, 56, 439–445.
- Wu, G.; Meininger, C.J.; Knabe, D.A.; Baze, F.W.; Rhoads, J.M. Arginine Nutrition in Development, Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 59–66.
- Lokhande, P.D.; Kuchekar, B.S.; Chabukswar, A.R.; Jagdale, S.C. Nitric Oxide: Role in Biological System. Asian J. Biochem. 2005, 1, 1–17.
- Garlichs, C.D.; Beyer, J.; Zhang, H.; Schmeisser, A.; Plötze, K.; Mügge, A.; Schellong, S.; Daniel, W.G. Decreased Plasma Concentrations of L-Hydroxy-Arginine as a Marker of Reduced NO Formation in Patients with Combined Cardiovascular Risk Factors. J. Lab. Clin. Med. 2000, 135, 419–425.
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17.
- Devés, R.; Boyd, C.A.R. Transporters for Cationic Amino Acids in Animal Cells: Discovery, Structure, and Function. Physiol. Rev. 1998, 78, 487–545.
- Böger, R.H.; Bode-Böger, S.M. The Clinical Pharmacology of L-Arginine. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 79–99.
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and Pharmacodynamic Properties of Oral L-Citrulline and L-Arginine: Impact on Nitric Oxide Metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59.
- Nakaki, T.; Hishikawa, K. The arginine paradox. Folia Pharmacol. Jpn. 2002, 119, 7–14.
- Li, C.; Huang, W.; Harris, M.B.; Goolsby, J.M.; Venema, R.C. Interaction of the Endothelial Nitric Oxide Synthase with the CAT-1 Arginine Transporter Enhances NO Release by a Mechanism Not Involving Arginine Transport. Biochem. J. 2005, 386, 567–574.
- Shin, S.; Mohan, S.; Fung, H.-L. Intracellular L-Arginine Concentration Does Not Determine NO Production in Endothelial Cells: Implications on the “L-Arginine Paradox”. Biochem. Biophys. Res. Commun. 2011, 414, 660–663.
- Aoyagi, K. Inhibition of Arginine Synthesis by Urea: A Mechanism for Arginine Deficiency in Renal Failure Which Leads to Increased Hydroxyl Radical Generation. In Guanidino Compounds in Biology and Medicine; Clark, J.F., Ed.; Springer: Boston, MA, USA, 2003; pp. 11–15.
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory Role for the Arginine–Nitric Oxide Pathway in Metabolism of Energy Substrates. J. Nutr. Biochem. 2006, 17, 571–588.
- Lee, J.; Ryu, H.; Ferrante, R.J.; Morris, S.M.; Ratan, R.R. Translational Control of Inducible Nitric Oxide Synthase Expression by Arginine Can Explain the Arginine Paradox. Proc. Natl. Acad. Sci. USA 2003, 100, 4843–4848.
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615.
- Shi, W.; Meininger, C.J.; Haynes, T.E.; Hatakeyama, K.; Wu, G. Regulation of Tetrahydrobiopterin Synthesis and Bioavailability in Endothelial Cells. Cell Biochem. Biophys. 2004, 41, 415–434.
- Böger, R.H. The Pharmacodynamics of L-Arginine. J. Nutr. 2007, 137, 1650S–1655S.
- Lorin, J.; Zeller, M.; Guilland, J.-C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and Nitric Oxide Synthase: Regulatory Mechanisms and Cardiovascular Aspects. Mol. Nutr. Food Res. 2014, 58, 101–116.
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975.
- Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 1798.
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94.
- Huynh, N.N.; Chin-Dusting, J. Amino Acids, Arginase and Nitric Oxide in Vascular Health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1–8.
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608.
- Flam, B.R.; Eichler, D.C.; Solomonson, L.P. Endothelial Nitric Oxide Production Is Tightly Coupled to the Citrulline–NO Cycle. Nitric Oxide 2007, 17, 115–121.
- Kedziora-Kornatowska, K.; Tkaczewski, W.; Blaszczyk, J.; Buczyn’ski, A.; Chojnacki, J.; Kedziora, J. Oxygen Metabolism in Blood of Patients with Gastric and Duodenal Ulcer Disease. Hepatogastroenterology 1995, 42, 246–249.
- Maher, T.J. L-Arginine. Continuing Education Module. New Hope Inst. Retail. 2000, 1–8.
- Merimee, T.J.; Rabinowitz, D.; Riggs, L.; Burgess, J.A.; Rimoin, D.L.; McKusick, V.A. Plasma Growth Hormone after Arginine Infusion: Clinical Experiences. N. Engl. J. Med. 1967, 276, 434–439.
- Kikuta, K.; Sawamura, T.; Miwa, S.; Hashimoto, N.; Masaki, T. High-Affinity Arginine Transport of Bovine Aortic Endothelial Cells Is Impaired by Lysophosphatidylcholine. Circ. Res. 1998, 83, 1088–1096.
- Kurose, I.; Wolf, R.; Grisham, M.B.; Aw, T.Y.; Specian, R.D.; Granger, D.N. Microvascular Responses to Inhibition of Nitric Oxide Production: Role of Active Oxidants. Circ. Res. 1995, 76, 30–39.
- Wu, G.; Meininger, C.J. Arginine Nutrition and Cardiovascular Function. J. Nutr. 2000, 130, 2626–2629.
- Preli, R.B.; Klein, K.P.; Herrington, D.M. Vascular Effects of Dietary L-Arginine Supplementation. Atherosclerosis 2002, 162, 1–15.
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-Arginine Therapy in Acute Myocardial Infarction: The Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) Randomized Clinical Trial. JAMA 2006, 295, 58.
- Adeghate, E.; Ponery, A.S.; El-Sharkawy, T.; Parvez, H. L-Arginine Stimulates Insulin Secretion from the Pancreas of Normal and Diabetic Rats. Amino Acids 2001, 21, 205–209.
- Pi, M.; Wu, Y.; Lenchik, N.I.; Gerling, I.; Quarles, L.D. GPRC6A Mediates the Effects of L-Arginine on Insulin Secretion in Mouse Pancreatic Islets. Endocrinology 2012, 153, 4608–4615.
- Smajilovic, S.; Clemmensen, C.; Johansen, L.D.; Wellendorph, P.; Holst, J.J.; Thams, P.G.; Ogo, E.; Bräuner-Osborne, H. The L-α-Amino Acid Receptor GPRC6A Is Expressed in the Islets of Langerhans but Is Not Involved in L-Arginine-Induced Insulin Release. Amino Acids 2013, 44, 383–390.
- Krause, M.S.; McClenaghan, N.H.; Flatt, P.R.; Homem de Bittencourt, P.I.; Murphy, C.; Newsholme, P. L-Arginine Is Essential for Pancreatic β-Cell Functional Integrity, Metabolism and Defense from Inflammatory Challenge. J. Endocrinol. 2011, 211, 87–97.
- Tsugawa, Y.; Handa, H.; Imai, T. Arginine Induces IGF-1 Secretion from the Endoplasmic Reticulum. Biochem. Biophys. Res. Commun. 2019, 514, 1128–1132.
- Cho, J.; Hiramoto, M.; Masaike, Y.; Sakamoto, S.; Imai, Y.; Imai, Y.; Handa, H.; Imai, T. UGGT1 Retains Proinsulin in the Endoplasmic Reticulum in an Arginine Dependent Manner. Biochem. Biophys. Res. Commun. 2020, 527, 668–675.
- Kohli, R.; Meininger, C.J.; Haynes, T.E.; Yan, W.; Self, J.T.; Wu, G. Dietary L-Arginine Supplementation Enhances Endothelial Nitric Oxide Synthesis in Streptozotocin-Induced Diabetic Rats. J. Nutr. 2004, 134, 600–608.
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary L-Arginine Supplementation Reduces Fat Mass in Zucker Diabetic Fatty Rats. J. Nutr. 2005, 135, 714–721.
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-Arginine Stimulates GLP-1 Secretion to Improve Glucose Tolerance in Male Mice. Endocrinology 2013, 154, 3978–3983.
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A. L-Arginine Ameliorates Oxidative Stress in Alloxan-Induced Experimental Diabetes Mellitus. J. Appl. Toxicol. 2004, 24, 93–97.
- del Carmen Ortiz, M.; Lores-Arnaiz, S.; Albertoni Borghese, M.F.; Balonga, S.; Lavagna, A.; Filipuzzi, A.L.; Cicerchia, D.; Majowicz, M.; Bustamante, J. Mitochondrial Dysfunction in Brain Cortex Mitochondria of STZ-Diabetic Rats: Effect of L-Arginine. Neurochem. Res. 2013, 38, 2570–2580.
- Pai, M.-H.; Huang, K.-H.; Wu, C.-H.; Yeh, S.-L. Effects of Dietary Arginine on Inflammatory Mediator and Receptor of Advanced Glycation Endproducts (RAGE) Expression in Rats with Streptozotocin-Induced Type 2 Diabetes. Br. J. Nutr. 2010, 104, 686–692.
- Wascher, T.C.; Graier, W.F.; Dittrich, P.; Hussain, M.A.; Bahadori, B.; Wallner, S.; Toplak, H. Effects of Low-Dose L-Arginine on Insulin-Mediated Vasodilatation and Insulin Sensitivity. Eur. J. Clin. Investig. 1997, 27, 690–695.
- Piatti, P.; Monti, L.D.; Valsecchi, G.; Magni, F.; Setola, E.; Marchesi, F.; Galli-Kienle, M.; Pozza, G.; Alberti, K.G.M.M. Long-Term Oral L-Arginine Administration Improves Peripheral and Hepatic Insulin Sensitivity in Type 2 Diabetic Patients. Diabetes Care 2001, 24, 875–880.
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial Effects of a Long-Term Oral L-Arginine Treatment Added to a Hypocaloric Diet and Exercise Training Program in Obese, Insulin-Resistant Type 2 Diabetic Patients. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E906–E912.
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-Arginine Supplementation Improves Endothelial Function and Ameliorates Insulin Sensitivity and Inflammation in Cardiopathic Nondiabetic Patients after an Aortocoronary Bypass. Metabolism 2009, 58, 1270–1276.
- Bogdanski, P.; Suliburska, J.; Grabanska, K.; Musialik, K.; Cieslewicz, A.; Skoluda, A.; Jablecka, A. Effect of 3-Month L-Arginine Supplementation on Insulin Resistance and Tumor Necrosis Factor Activity in Patients with Visceral Obesity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 816–823.
- Jabłecka, A.; Bogda, P.; Balcer, N.; Cie, A.; Skołuda, A.; Musialik, K. The Effect of Oral L-Arginine Supplementation on Fasting Glucose, HbA1c, Nitric Oxide and Total Antioxidant Status in Diabetic Patients with Atherosclerotic Peripheral Arterial Disease of Lower Extremities. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 342–350.
- Bogdanski, P.; Szulinska, M.; Suliburska, J.; Pupek-Musialik, D.; Jablecka, A.; Witmanowski, H. Supplementation with L-Arginine Favorably Influences Plasminogen Activator Inhibitor Type 1 Concentration in Obese Patients. A Randomized, Double Blind Trial. J. Endocrinol. Investig. 2013, 36, 221–226.
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Pupek-Musialik, D.; Jablecka, A. Changes in Mineral Status Are Associated with Improvements in Insulin Sensitivity in Obese Patients Following L-Arginine Supplementation. Eur. J. Nutr. 2014, 53, 387–393.
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-Arginine Enriched Biscuits Improve Endothelial Function and Glucose Metabolism: A Pilot Study in Healthy Subjects and a Cross-Over Study in Subjects with Impaired Glucose Tolerance and Metabolic Syndrome. Metabolism 2013, 62, 255–264.
- Monti, L.D.; Setola, E.; Lucotti, P.C.G.; Marrocco-Trischitta, M.M.; Comola, M.; Galluccio, E.; Poggi, A.; Mammì, S.; Catapano, A.L.; Comi, G.; et al. Effect of a Long-Term Oral L-Arginine Supplementation on Glucose Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes. Metab. 2012, 14, 893–900.
- Monti, L.D.; Galluccio, E.; Villa, V.; Fontana, B.; Spadoni, S.; Piatti, P.M. Decreased Diabetes Risk over 9 Year after 18-Month Oral L-Arginine Treatment in Middle-Aged Subjects with Impaired Glucose Tolerance and Metabolic Syndrome (Extension Evaluation of L-Arginine Study). Eur. J. Nutr. 2018, 57, 2805–2817.
- Assumpção, C.R.L.; Brunini, T.M.C.; Pereira, N.R.; Godoy-Matos, A.F.; Siqueira, M.A.S.; Mann, G.E.; Mendes-Ribeiro, A.C. Insulin Resistance in Obesity and Metabolic Syndrome: Is There a Connection with Platelet L-Arginine Transport? Blood Cells. Mol. Dis. 2010, 45, 338–342.
- Rajapakse, N.W.; Chong, A.L.; Zhang, W.-Z.; Kaye, D.M. Insulin-Mediated Activation of the L-Arginine Nitric Oxide Pathway in Man, and Its Impairment in Diabetes. PLoS ONE 2013, 8, e61840.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
07 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




