
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodica Olar | + 3999 word(s) | 3999 | 2022-02-08 09:24:42 | | | |
| 2 | Lindsay Dong | Meta information modification | 3999 | 2022-03-08 04:54:52 | | | | |
| 3 | Lindsay Dong | Meta information modification | 3999 | 2022-03-08 04:58:44 | | |
Video Upload Options
Microbial biofilms are represented by sessile microbial communities with modified gene expression and phenotype, adhered to a surface and embedded in a matrix of self-produced extracellular polymeric substances (EPS). Microbial biofilms can develop on both prosthetic devices and tissues, generating chronic and persistent infections that cannot be eradicated with classical organic-based antimicrobials, because of their increased tolerance to antimicrobials and the host immune system. Several complexes based mostly on 3d ions have shown promising potential for fighting biofilm-associated infections, due to their large spectrum antimicrobial and anti-biofilm activity. The literature usually reports species containing Mn(II), Ni(II), Co(II), Cu(II) or Zn(II) and a large variety of multidentate ligands with chelating properties such as antibiotics, Schiff bases, biguanides, N-based macrocyclic and fused rings derivatives.
1. Introduction
2. Complexes with Antibiofilm Activity
2.1. Complexes with Antibiotics
2.2. Complexes with Heterocyclic Derivatives
2.3. Complexes with Schiff Bases
2.4. Complexes with Biguanide Derivatives
2.5. Complexes with Macrocyclic Ligands
2.6. Complexes with Miscellaneous Ligands
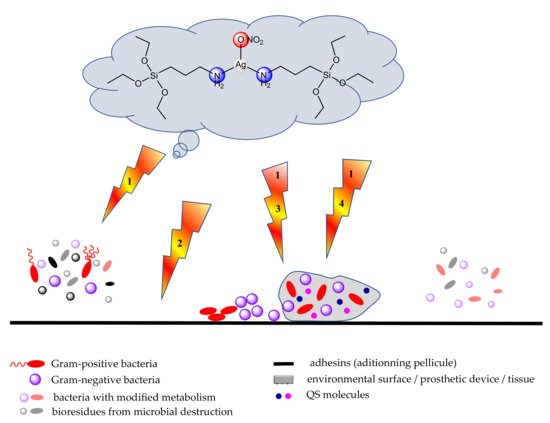
2.7. Materials as Carriers for Metal Ions or Complexes with Anti-Biofilm Activity
In order to overcome the problems associated with the use of antibiotics, some polymer species complexed with proper metal ions or loaded with biological active complexes as well as nanomaterials with anti-biofilm properties and biocompatibility/environmental safety have also been developed. Several dendrimers and polymers appropriately modified with coordinative groups able to chelate metal ions were designed for this purpose. Moreover, several attempts were made for the complexes’ incorporation into organic or inorganic matrices.
3. Conclusions
One of the most promising leads for the design of new complexes with anti-biofilm activity are the redox active metal ions such Cu(II), Fe(III) and Mn(II) but the less-studied ones such VO(IV) and Ru(II) should be also considered. All of these ions have ROS or NOS generation as a common mechanism of action. The best anti-biofilm activity is achieved then these ions are combined with multidentate ligands, especially bearing N as donor atoms, assuring enhanced stability. Furthermore, the perchlorate anion that easily generates single crystals seems to enhance the anti-biofilm activity in complexes bearing neutral organic ligands. The most active compounds show an improved activity after incorporation in organic, inorganic or composite matrices. The majority of the current literature refers to the in vitro study of the anti-biofilm activity of complexes, this explaining the paucity of novel anti-biofilm agents in medical practice. Thus, there is an urgent need for additional in vivo studies in this field in order to elucidate the safety, efficacy and toxicity of these species in order to develop new valuable drugs for the treatment of biofilm-associated infections.
References
- Kamaruzzaman, N.F.; Tan, L.P.; Yazid, K.A.M.; Saeed, S.I.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Chivu, A.; Gibson, A.J. Targeting the Bacterial Protective Armour; Challenges and Novel Strategies in the Treatment of Microbial Biofilm. Materials 2018, 11, 1705.
- Pircalabioru, G.G.; Chifiriuc, M.C. Nanoparticulate drug-delivery systems for fighting microbial biofilms–from bench to bedside. Future Microbiol. 2020, 15, 679–698.
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida–streptococcal interactions in biofilm associated oral diseases. PLoS Pathog. 2018, 14, e1007342.
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in Implant Infections: Its Production and Regulation. Int. J. Artif. Organ. 2005, 28, 1062–1068.
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 248, 1318–1322.
- Martin, C.; Low, W.; Gupta, A.; Amin, M.; Radecka, I.; Britland, S.; Raj, P.; Kenward, K. Strategies for Antimicrobial Drug Delivery to Biofilm. Curr. Pharm. Des. 2014, 21, 43–66.
- Mihai, M.M.; Preda, M.; Lungu, I.; Cartelle Gestal, M.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179.
- Pallavicini, P.; Dacarro, G.; Diaz-Fernandez, Y.A.; Taglietti, A. Coordination chemistry of surface-grafted ligands for antibacterial materials. Coord. Chem. Rev. 2014, 275, 37–53.
- Regiel-Futyra, A.; Dąbrowski, J.M.; Mazuryk, O.; Śpiewak, K.; Kyzioł, A. Bioinorganic antimicrobial strategies in the resistance era. Coord. Chem. Rev. 2017, 351, 76–117.
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332.
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175.
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509.
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958.
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208.
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547.
- Gomes, I.B.; Simões, M.; Simões, L.C. Copper Surfaces in Biofilm Control. Nanomaterials 2020, 10, 2491.
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384.
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. FEBS J. 2017, 284, 3662–3683.
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490.
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137.
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104.
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10.
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405.
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526.
- Tewes, F.; Bahamondez-Canas, T.F.; Smyth, H.D.C. Efficacy of Ciprofloxacin and Its Copper Complex against Pseudomonas aeruginosa Biofilms. AAPS PharmSciTech 2019, 20, 205.
- Rafiee, F.; Haghi, F.; Bikas, R.; Heidari, A.; Gholami, M.; Kozakiewicz, A.; Zeighami, H. Synthesis, characterization and assessment of anti-quorum sensing activity of copper(II)-ciprofloxacin complex against Pseudomonas aeruginosa PAO1. AMB Expr. 2020, 10, 82.
- Stevanović, N.L.J.; Aleksic, I.; Kljun, J.; Skaro Bogojevic, S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B.Ð. Copper(II) and Zinc(II) Complexes with the Clinically Used Fluconazole: Comparison of Antifungal Activity and Therapeutic Potential. Pharmaceuticals 2021, 14, 24.
- Calu, L.; Badea, M.; Cerc Korošec, R.; Bukovec, P.; Daniliuc, C.C.; Chifiriuc, M.C.; Măruţescu, L.; Ciulică, C.; Şerban, G.; Olar, R. Thermal behaviour of some novel biologically active complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 697–708.
- Olar, R.; Calu, L.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Velescu, B.; Stoica, O.; Ioniţă, G.; Stanică, N.; Silvestro, L.; et al. Thermal behaviour of some biologically active species based on complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 685–696.
- Badea, M.; Calu, L.; Celan Korosin, N.; David, I.G.; Chifiriuc, M.C.; Bleotu, C.; Ioniţă, G.; Silvestro, L.; Maurer, M.; Olar, R. Thermal behaviour of some biological active perchlorate complexes with a triazolopyrimidine derivative. J. Therm. Anal. Calorim. 2018, 134, 665–677.
- Ruta, L.L.; Farcasanu, I.C.; Bacalum, M.; Raileanu, M.; Rostas, A.M.; Daniliuc, C.G.; Chifiriuc, M.C.; Maru-tescu, L.; Popa, M.; Badea, M.; et al. Biological activity of N-N-helating heterocycle copper(II) complexes modulated by an auxiliary triazolopyrimidine ligand. Molecules 2021, 26, 6772.
- Badea, M.; Vlaicu, I.D.; Olar, R.; Constand, M.; Bleotu, C.; Chifiriuc, M.C.; Marutescu, L.; Lazar, V.; Grecu, M.N.; Marinescu, D. Thermal behaviour and characterisation of new biologically active Cu(II) complexes with benzimidazole as main ligand. J. Therm. Anal. Calorim. 2014, 118, 1119–1133.
- Vlaicu, I.D.; Constand, M.; Olar, R.; Marinescu, D.; Grecu, M.N.; Lazar, V.; Chifiriuc, M.C.; Badea, M. Thermal stability of new biologic active copper(II) complexes with 5,6-dimethylbenzimidazole. J. Therm. Anal. Calorim. 2013, 113, 1369–1377.
- Glišić, B.Đ.; Aleksic, I.; Comba, P.; Wadepohl, H.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Djuran, M.I. Copper(II) complexes with aromatic nitrogen-containing heterocycles as effective inhibitors of quorum sensing activity in Pseudomonas aeruginosa. RSC Advances 2013, 6, 86695–86709.
- Al-Shabib, N.A.; Husain, F.M.; Khan, R.A.; Khan, M.S.; Alam, M.Z.; Ansari, F.A.; Laeeq, S.; Zubair, M.; Shahzad, S.A.; Khan, J.M.; et al. Interference of phosphane copper (I) complexes of b-carboline with quorum sensing regulated virulence functions and biofilm in foodborne pathogenic bacteria: A first report. Saudi J. Biol. Sci. 2019, 26, 308–316.
- Bernardi, T.; Badel, S.; Mayer, P.; Groelly, J.; de Frémont, P.; Jacques, B.; Braunstein, P.; Teyssot, M.-L.; Gaulier, C.; Cisnetti, F.; et al. High-Throughput Screening of Metal-N-Heterocyclic Carbene Complexes against Biofilm Formation by Pathogenic Bacteria. Chem. Med. Chem. 2014, 9, 1140–1144.
- Pan, T.; Wang, Y.; Liu, F.-S.; Lin, H.; Zhou, Y. Copper(I)–NHCs complexes: Synthesis, characterization and their inhibition against the biofilm formation of Streptococcus mutans. Polyhedron 2021, 197, 115033.
- Soto-Aguilera, N.; Üstün, E.; Tutar, U.; Çelik, C.; Gürbüz, N.; Özdemir, İ. Antimicrobial activity, inhibition of biofilm formation, and molecular docking study of novel Ag-NHC complexes. J. Organomet. Chem. 2021, 954–955, 122082.
- Fu, D.; Yang, S.; Lu, J.; Lian, H.; Qin, K. Two Cu(II) Coordination Polymers: Treatment Activity on Spine Surgery Incision Infection by Inhibiting the Staphylococcus aureus Biofilm Formation. J. Clust. Sci. 2021, 1–8.
- Olar, R.; Badea, M.; Bacalum, M.; Raileanu, M.; Ruta, L.L.; Farcasanu, I.C.; Rostas, A.M.; Vlaicu, I.D.; Popa, M.; Chifiriuc, M.C. Antiproliferative and antibacterial properties of biocompatible copper(II) complexes bearing chelating N,N-heterocycle ligands and potential mechanisms of action. Biometals 2021, 34, 1155–1172.
- Rostas, A.M.; Badea, M.; Ruţă, L.L.; Farcaşanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Čelan Korošin, N.; Cerc Korošec, R.; et al. Copper(II) complexes with mixed heterocycle ligands as promising antibacterial and antitumor species. Molecules 2020, 25, 3777.
- Zandvakili, T.; Fatemi, S.J.; Ebrahimipour, S.Y.; Ebrahimnejad, H.; Castro, J.; Dusek, M.; Eigner, V. Deferasirox pyridine solvate and its Cu(II) complex: Synthesis, crystal structure, Hirshfeld surface analysis, antimicrobial assays and antioxidant activity. J. Mol. Struct. 2022, 1249, 131525.
- Calu, L.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; David, G.-I.; Ioniţă, G.; Măruțescu, L.; Lazăr, V.; Stănică, N.; Soponaru, I.; et al. Synthesis, spectral, thermal, magnetic and biological characterization of Co(II), Ni(II), Cu(II) and Zn(II) complexes with a Schiff base bearing a 1,2,4-triazole pharmacophore. J. Therm. Anal. Calorim. 2014, 120, 375–386.
- Badea, M.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Marin, A.; Ion, S.; Ioniță, G.; Stănică, N.; Măruțescu, L.; Lazăr, V.; et al. Thermal behaviour of some novel antimicrobials based on complexes with a Schiff base bearing 1,2,4-triazole pharmacophore. J. Therm. Anal. Calorim. 2014, 118, 1145–1157.
- Calu, L.; Badea, M.; Čelan Korošin, N.; Chifiriuc, M.C.; Bleotu, C.; Stanică, N.; Silvestro, L.; Maurer, M.; Olar, R. Spectral, thermal and biological characterization of complexes with a Schiff base bearing triazole moiety as potential antimicrobial species. J. Therm. Anal. Calorim. 2018, 134, 1839–1850.
- Reiss, A.; Chifiriuc, M.C.; Amzoiu, E.; Spînu, C.I. Transition metal(II) complexes with cefotaxime-derived Schiff base: Synthesis, characterization and antimicrobial studies. Bioinorg. Chem. Appl. 2014, 2014, 1–17.
- Reiss, A.; Chifiriuc, M.C.; Amzoiu, E.; Cioateră, N.; Dăbuleanu, I.; Rotaru, P. New metal(II) complexes with ceftazidime Schiff base. J. Therm. Anal. Calorim. 2017, 131, 2073–2085.
- Zarafu, I.; Badea, M.; Ioniţă, G.; Ioniţă, P.; Păun, A.; Bucur, M.; Chifiriuc, M.C.; Bleotu, C.; Olar, R. Spectral, magnetic, thermal and biological studies on Ca(II) and Cu(II) complexes with a novel crowned Schiff base. J. Therm. Anal. Calorim. 2016, 127, 1511–1521.
- Zarafu, I.; Olar, R.; Chifiriuc, M.C.; Bleotu, C.; Ioniţă, P.; Mulţescu, M.; Ioniță, G.; Grădișteanu, G.; Tatibouët, A.; Badea, M. Synthesis, thermal, spectral, antimicrobial and cytotoxicity profile of the Schiff bases bearing pyrazolone moiety and their Cu(II) complexes. J. Therm. Anal. Calorim. 2018, 134, 1851–1861.
- Zarafu, I.; Badea, M.; Ioniţă, G.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Ioniţă, P.; Tatibouët, A.; Olar, R. Thermal, spectral and biological characterisation of copper(II) complexes with isoniazid-based hydrazones. J. Therm. Anal. Calorim. 2019, 136, 1977–1987.
- Usman, M.; Arjmand, F.; Ahmad, M.; Khan, M.S.; Ahmad, I.; Tabassum, S. A comparative analyses of bioactive Cu(II) complexes using Hirshfeld surface and density functional theory (DFT) methods: DNA binding studies, cleavage and antibiofilm activities. Inorg. Chim. Acta 2016, 453, 193–201.
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Mohamadi, M.; Suarez, S.; Baggio, R.; Khaleghi, M.; Torkzadeh-Mahani, M.; Mostafavi, A. Synthesis, characterization, X-ray crystal structure, DFT calculation, DNA binding, and antimicrobial assays of two new mixed-ligand copper(II) complexes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 142, 410–422.
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, C.-M.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.E.; Bucur, M.; Lazar, V.; Finaru, A. Prospects for new antimicrobials based on N,N-dimethylbiguanide complexes as effective agents on both planktonic and adhered microbial strains. Eur. J. Med. Chem. 2010, 45, 2868–2875.
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, M.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.; Lazar, V. N,N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 3027–3034.
- Olar, R.; Badea, M.; Marinescu, D.; Iorgulescu, E.E.; Frunza, E.; Lazar, V.; Chifiriuc, C. Thermal, spectral and antimicrobial study on some Cu(II) complexes with ligands bearing biguanide moieties. J. Therm. Anal. Calorim. 2009, 99, 815–821.
- Maxim, C.; Badea, M.; Rostas, A.M.; Chifiriuc, M.C.; Gradisteanu Pircalabioru, G.; Avram, S.; Olar, R. Copper(II) species with 1-(o-tolyl)biguanide: Structural characterization, ROS scavenging, antibacterial activity, biocompatibility and in silico studies. Appl. Organomet. Chem. 2021, e6471.
- Nuţă, I.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Daniliuc, C.-G.; Olar, R. Synthesis, physico-chemical characterization and bioevaluation of Ni(II), Pd(II), and Pt(II) complexes with 1-(o-tolyl)biguanide: Antimicrobial and antitumor studies. Appl. Organ. Chem. 2020, 34, e5807.
- Mihalache, M.; Oprea, O.; Guran, C.; Holban, A.M. Synthesis, characterization, and biological activity of some complex combinations of nickel with α-ketoglutaric acid and 1-(o-tolyl)biguanide. Comptes Rendus Chim. 2018, 21, 32–40.
- Mihalache, M.; Guran, C.; Meghea, A.; Bercu, V.; Motelica, L.; Holban, A.M. Complexes of Cu (II) with α-Ketoglutaric Acid and 1-(o-tolyl)Biguanide. Synthesis, Characterization and Biological Activity. Rev. Chim. 2019, 70, 3603–3610.
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344.
- Badea, M.; Grecu, M.N.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Iorgulescu, E.E.; Avram, S.; Uivarosi, V.; Munteanu, A.-C.; Ghica, D.; et al. Insight on Ni(II) and Cu(II) complexes of biguanide derivatives developed as effective antimicrobial and antitumor agents. Appl. Organomet. Chem. 2021, 35, e6155.
- Olar, R.; Pătraşcu, F.; Chifiriuc, M.C.; Iorgulescu, E.E.; Bleotu, C.; Măruţescu, L.; Lazăr, V.; Marinescu, D.; Stănică, N.; Badea, M. Insight on thermal, spectral, magnetic and biological behaviour of new Ni(II), Cu(II) and Zn(II) complexes with a pentaazamacrocyclic ligand derived from nicotinamide. J. Therm. Anal. Calorim. 2014, 118, 1159–1168.
- Badea, M.; Pătraşcu, F.; Cerc Korošec, R.; Bukovec, P.; Raita, M.; Chifiriuc, M.C.; Măruțescu, L.; Bleotu, C.; Velescu, B.; Marinescu, D.; et al. Thermal, spectral, magnetic and biologic characterization of new Ni(II), Cu(II) and Zn(II) complexes with a hexaazamacrocyclic ligand bearing ketopyridine moieties. J. Therm. Anal. Calorim. 2014, 118, 1183–1193.
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper (II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 2018, 8, 8050.
- Vázquez-Armenta, F.J.; Beltrán-Torres, M.; Ayala-Zavala, J.F.; Velázquez-Contreras, E.F.; Rocha-Alonzo, F.; González-Aguilar, G.A.; Sugich-Miranda, R. Antibiofilm properties of copper (II) and iron (III) complexes with an EDTA-based phenylene macrocycle and its acyclic analogue against food and clinical related pathogens. Polyhedron 2021, 198, 115076.
- Bucur, C.; Badea, M.; Larisa, C.; Marinescu, D.; Grecu, M.N.; Stanica, N.; Chifiriuc, M.C.; Olar, R. Thermal behaviour of some new complexes with decaaza bismacrocyclic ligand as potential antimicrobial species. J. Therm. Anal. Calorim. 2012, 110, 235–241.
- Bucur, C.; Korošec, R.C.; Badea, M.; Calu, L.; Chifiriuc, M.C.; Grecu, N.; Stănică, N.; Marinescu, D.; Olar, R. Investigation of thermal stability, spectral, magnetic, and antimicrobial behavior for new complexes of Ni(II), Cu(II), and Zn(II) with a bismacrocyclic ligand. J. Therm. Anal. Calorim. 2013, 113, 1287–1295.
- Badea, M.; Bucur, C.; Chifiriuc, M.C.; Bleotu, C.; Grecu, M.-N.; Lazar, V.; Marinescu, D.; Olar, R. Insight on thermal behaviour of new complexes of Ni(II), Cu(II) and Zn(II) with a bismacrocyclic ligand developed as biologically active species. J. Therm. Anal. Calorim. 2016, 127, 487–497.
- Bucur, C.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Iorgulescu, E.E.; Badea, I.A.; Grecu, M.N.; Lazăr, V.; Patriciu, O.-I.; Marinescu, D.; et al. Studies on thermal, spectral, magnetic and biological properties of new Ni(II), Cu(II) and Zn(II) complexes with a bismacrocyclic ligand bearing an aromatic linker. J. Therm. Anal. Calorim. 2013, 115, 2179–2189.
- Bano, N.; Rauf, M.A.; Owais, M.; Shakir, M. Pharmacologically bio-relevant N-functionalized homo-binuclear macrocyclic complexes: Synthesis, spectral studies, biological screening, HSA binding, and molecular docking. Inorg. Nano-Met. Chem. 2019, 49, 413–430.
- Gholami, M.; Zeighami, H.; Bikas, R.; Heidari, A.; Rafiee, F.; Haghi, F. Inhibitory activity of metal-curcumin complexes on quorum sensing related virulence factors of Pseudomonas aeruginosa PAO1. AMB Express 2020, 10, 111.
- Rubab, M.; Akhtar, M.N.; Zierkiewicz, W.; Michalczyk, M.; Nadeem, R.; Shahid, M.; Tahir, M.N.; Akram, M.; Hanif, M.A.; AlDamen, M.A. The role of hydrogen bonding in π···π stacking interactions in Ni(II) complex derived from triethanolamine: Synthesis, crystal structure, antimicrobial, and DFT studies. Res. Chem. Intermed. 2019, 45, 5649–5664.
- Drzewiecka-Antonik, A.; Rejmak, P.; Klepka, M.T.; Wolska, A.; Pietrzyk, P.; Stępień, K.; Sanna, G.; Struga, M. Synthesis, structural studies and biological activity of novel Cu(II) complexes with thiourea derivatives of 4-azatricyclodec-8-ene-3,5-dione. J. Inorg. Biochem. 2017, 176, 8–16.
- Bukonjić, A.M.; Tomović, D.L.; Nikolić, M.V.; Mijajlović, M.Ž.; Jevtić, V.V.; Ratković, Z.R.; Novaković, S.B.; Bogdanović, G.A.; Radojević, I.D.; Maksimović, J.Z.; et al. Antibacterial, antibiofilm and antioxidant screening of copper(II)-complexes with some S-alkyl derivatives of thiosalicylic acid. Crystal structure of the binuclear copper(II)-complex with S-propyl derivative of thiosalicylic acid. J. Mol. Struct. 2017, 1128, 330–337.
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyngd, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, structural and antimicrobial studies of type II topoisomerase-targeted copper(II) complexes of 1,3-disubstituted thiourea ligands. J. Inorg. Biochem. 2018, 182, 61–70.
- Maruțescu, L.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Daniliuc, C.-G.; Falcescu, D.; Kamerzan, C.M.; Badea, M.; Olar, R. Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolopyrimidine. Molecules 2017, 22, 1233.
- Boni Dias, B.; Gomes da Silva Dantas, F.; Galvão, F.; Cupozak-Pinheiro, W.J.; Wender, H.; Pizzuti, L.; Rosa, P.P.; Tenório, K.V.; Gatto, C.C.; Negri, M.; et al. Synthesis, structural characterization, and prospects for new cobalt (II) complexes with thiocarbamoyl-pyrazoline ligands as promising antifungal agents. J. Inorg. Biochem. 2020, 213, 111277.
- Borges, A.; Simões, M.; Todorović, T.R.; Filipović, N.R.; García-Sosa, A.T. Cobalt Complex with Thiazole-Based Ligand as New Pseudomonas aeruginosa Quorum Quencher, Biofilm Inhibitor and Virulence Attenuator. Molecules 2018, 23, 1385.
- Hopa, C.; Kara, H.; Aybey, A. Synthesis, structural characterization and biological evaluation of novel mixed-ligand Co(II) complexes as quorum sensing inhibitory agent. J. Mol. Struct. 2020, 1202, 127322.
- Akhtar, M.N.; Harrison, W.T.A.; Shahid, M.; Khan, I.-U.; Ejaz, I.-U.K.; Iqbal, J. Synthesis, crystal structure and biological activity of a cobalt(II) complex of N,N,N′,N′-tetrakis(2-hydroxypropyl) ethylenediamine. Trans. Met. Chem. 2016, 41, 325–330.
- Di Santo, A.; Gil, D.M.; Pomiro, F.; Piro, O.E.; Echeverría, G.A.; Arena, M.; Luciardi, C.; Carbonio, R.E.; Ben Altabef, A. Biofilm inhibition by a new Mn(II) complex with sulfamethoxazole: Synthesis, spectroscopic characterization and crystal structure. Inorg. Chim. Acta 2015, 436, 16–22.
- Khan, M.S.; Hayat, M.U.; Khanam, M.; Saeed, H.; Owais, M.; Khalid, M.; Shahid, M.; Ahmad, M. Role of biologically important imidazole moiety on the antimicrobial and anticancer activity of Fe(III) and Mn(II) complexes. J. Biomol. Struct. Dyn. 2021, 39, 4037–4050.
- Jabłońska-Wawrzycka, A.; Rogala, P.; Czerwonka, G.; Michałkiewicz, S.; Hodorowicz, M.; Gałczyńska, K.; Cieślak, B.; Kowalczyk, P. Tuning Anti-Biofilm Activity of Manganese(II) Complexes: Linking Biological Effectiveness of Heteroaromatic Complexes of Alcohol, Aldehyde, Ketone, and Carboxylic Acid with Structural Effects and Redox Activity. Int. J. Mol. Sci. 2021, 22, 4847.
- Chen, X.-Y.; Ji, P. A Microporous Zn(II)–MOF for Solvent-Free Cyanosilylation and Treatment Effect Against Bacterial Infection on Burn Patients Via Inhibiting the Staphylococcus aureus Biofilm Formation. J. Inorg. Organ. Polym. Mater. 2021, 31, 492–499.
- Nayak, M.; Kumar Singh, A.; Prakash, P.; Kant, R.; Bhattacharya, S. Strucutral studies on thiosalicylate complexes of Zn(II) & Hg(II). First insight into Zn(II)-thiosalicylate complex as potential antibacterial, antibiofilm and anti-tumour agent. Inorg. Chim. Acta 2020, 501, 119263.
- Maurya, V.K.; Singh, A.K.; Singh, R.P.; Yadav, S.; Kumar, K.; Prakash, P.; Prasad, L.B. Synthesis and evaluation of Zn(II) dithiocarbamate complexes as potential antibacterial, antibiofilm, and antitumor agents. J. Coord. Chem. 2019, 72, 3338–3358.
- Beeton, M.L.; Aldrich-Wright, J.R.; Bolhuis, A. The antimicrobial and antibiofilm activities of copper(II) complexes. J. Inorg. Biochem. 2014, 140, 167–172.
- Badea, M.; Iosub, E.; Chifiriuc, C.M.; Marutescu, L.; Iorgulescu, E.E.; Lazar, V.; Marinescu, D.; Bleotu, C.; Olar, R. Thermal, spectral, electrochemical and biologic characterization of new Pd(II) complexes with ligands bearing biguanide moieties. J. Therm. Anal. Calorim. 2012, 111, 1753–1761.
- Cordenonsi Bonez, P.; Agertt, V.A.; Guidolin Rossi, G.; dos Santos Siqueira, F.; Siqueira, J.D.; Marques, L.L.; Manzoni de Oliveira, G.N.; Vianna Santos, R.C.; Anraku de Campos, M.M. Sulfonamides complexed with metals as mycobacterial biofilms inhibitors. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100217.
- Mizdal, C.; Stefanello, S.; Bonez, P.; Agertt, V.; Flores, V.; Rossi, G.; Siqueira, F.; Marques, L.; Campos, M. Anti-biofilm and Antibacterial Effects of Novel Metal-coordinated Sulfamethoxazole Against Escherichia coli. Lett. Drug Des. Discov. 2017, 14, 339–344.
- Matiadis, D.; Karagiaouri, M.; Mavroidi, B.; Nowak, K.E.; Katsipis, G.; Pelecanou, M.; Pantazaki, A.; Sagnou, M. Synthesis and antimicrobial evaluation of a pyrazolinepyridine silver(I) complex: DNA-interaction and antibiofilm activity. Biometals 2021, 34, 67–85.
- Ventura, R.F.; Galdino, A.C.M.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A.P.F. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710.
- Mihsen, H.H.; Shareef, N.K.; Alwazni, W.S. Synthesis, Characterization and Antibacterial Studies of Silver Complex of 3-Aminopropyltriethoxysilane. Asian J. Chem. 2018, 30, 1465–1468.
- Klrissa, S.; Katouli, M. Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect. Epidemiol. Med. 2016, 2, 25–32.
- Bahamondez-Canas, T.F.; Zhang, H.; Tewes, F.; Leal, J.; Smyth, H.D. PEGylation of tobramycin improves mucus penetration and antimicrobial activity against Pseudomonas aeruginosa biofilms in vitro. Mol. Pharm. 2018, 15, 1643–1652.
- Hubberstey, P.; Suksangpanya, U. Hydrogen-Bonded Supramolecular Chain and Sheet Formation by Coordinated Guanidine Derivatives. Struct. Bond. 2004, 111, 33–83.
- Gabel, S.A.; Duff, M.R.; Pedersen, L.C.; DeRose, E.F.; Krahn, J.M.; Howell, E.E.; London, R.E. A Structural Basis for Biguanide Activity. Biochemistry 2017, 56, 4786–4798.
- Yu, H.; Liu, L.; Yang, H.; Zhou, R.; Che, C.; Li, X.; Li, C.; Luan, S.; Yin, J.; Shi, H. Water-Insoluble Polymeric Guanidine Derivative and Application in the Preparation of Antibacterial Coating of Catheter. ACS Appl. Mater. Interfaces 2018, 10, 39257–39267.
- Li, J.; Zhong, W.; Zhang, K.; Wang, D.; Hu, J.; Chan-Park, M.B. Biguanide-Derived Polymeric Nanoparticles Kill MRSA Biofilm and Suppress Infection In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 21231.
- Arouri, A.; Essghaier, B.; Dridi, R.; Zid, M.F. Crystal structure, spectral investigation, thermal properties and evaluation of the antimicrobial behavior of a new 2D polymeric Mn(II): (C6H9N2)2·H2O. J. Mol. Struct. 2021, 1244, 131251.
- Essghaier, B.; Dridi, R.; Arouri, A.; Zid, M.F. Synthesis, structural characterization and prospects for a new tris (5-methylbenzimidazole) tris (oxalato) ferrate(III) trihydrate complex as a promising antibacterial and antifungal agent. Polyhedron 2021, 208, 115420.
- Li, J.-Y.; Li, B.; Liu, X.-M. A New Co(II)-Based Metal–Organic Framework: Photocatalytic Dye Degradation and Treatment Activity Against Renal Failure Patients Combined with Staphylococcus aureus Biofilm Formation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1836–1845.
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830.
- Bosch, P.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Kukeva, R.; Stoyanova, R.; Grabchev, I. New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials 2019, 12, 3020.
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Synthesis, spectral characterization and in vitro antimicrobial activity in liquid medium and applied on cotton fabric of a new PAMAM metallodendrimer. Int. J. Polym. Anal. Char. 2018, 23, 45–57.
- Grabcheva, I.; Vasileva-Tonkovab, E.; Stanevac, D.; Boschd, P.; Kukevae, R.; Stoyanova, R. Impact of the Cu(II) and Zn(II) ions on the functional properties of new PAMAM metallodendrimers. New J. Chem. 2018, 42, 7853–7862.
- Llamazares, C.; Sanz del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; Javier de la Mata, F.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405.
- Wonoputri, V.; Gunawan, C.; Liu, S.; Barraud, N.; Yee, L.H.; Lim, M.; Amal, R. Copper Complex in Poly(vinyl chloride) as a Nitric Oxide-Generating Catalyst for the Control of Nitrifying Bacterial Biofilms. ACS Appl. Mater. Interfaces 2015, 7, 22148–22156.
- Saleh, S.; Sweileh, B.; Taha, S.O.; Mahmoud, R.; Taha, M.O. Preparation of Polyester-Based Metal-Cross Linked Polymeric Composites as Novel Materials Resistant to Bacterial Adhesion and Biofilm Formation. Molecules 2011, 16, 933–950.
- Cai, L.; Huang, Y.; Duan, Y.; Liu, Q.; Xu, Q.; Jia, J.; Wang, J.; Tong, Q.; Luo, P.; Wen, Y.; et al. Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes. Nano Res. 2021, 14, 2735–2748.
- Ma, H.; Darmawan, E.T.; Zhang, M.; Zhang, L.; Bryers, J.D. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J. Control. Release 2013, 172, 1035–1044.
- Quiros, J.; Boltes, K.; Aguado, S.; de Villoria, R.G.; Vilatela, J.J.; Rosal, R. Antimicrobial metal-organic frameworks incorporated into electrospun fibers. Chem. Eng. J. 2015, 262, 189–197.
- Duan, F.; Feng, X.; Jin, Y.; Liu, D.; Yang, X.; Zhou, G.; Liu, D.; Li, Z.; Liang, X.-J.; Zhang, J. Metal-carbenicillin framework-based nanoantibiotics with enhanced penetration and highly efficient inhibition of MRSA. Biomaterials 2017, 144, 155–165.




