Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dhouha Dridi | + 1675 word(s) | 1675 | 2022-02-22 03:07:13 | | | |
| 2 | Bruce Ren | + 3 word(s) | 1678 | 2022-02-28 03:21:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dridi, D. Umbilical Endometriosis and Pathogenic Theory Proposal. Encyclopedia. Available online: https://encyclopedia.pub/entry/19940 (accessed on 07 February 2026).
Dridi D. Umbilical Endometriosis and Pathogenic Theory Proposal. Encyclopedia. Available at: https://encyclopedia.pub/entry/19940. Accessed February 07, 2026.
Dridi, Dhouha. "Umbilical Endometriosis and Pathogenic Theory Proposal" Encyclopedia, https://encyclopedia.pub/entry/19940 (accessed February 07, 2026).
Dridi, D. (2022, February 26). Umbilical Endometriosis and Pathogenic Theory Proposal. In Encyclopedia. https://encyclopedia.pub/entry/19940
Dridi, Dhouha. "Umbilical Endometriosis and Pathogenic Theory Proposal." Encyclopedia. Web. 26 February, 2022.
Copy Citation
Endometriosis is a benign gynecological disorder that affects about 5% of reproductive-aged women. The pelvic cavity is the most common location of endometriotic implants, but about 12% of lesions are extragenital and, among the extra-pelvic sites, endometriosis of the abdominal wall (AWE) is the most common. Umbilical endometriosis (UE), or Villar’s nodule, as first described by Villar in 1886, is defined as the presence of endometrial glands and/or stroma within the umbilicus.
endometriosis
umbilical endometriosis
Villar’s nodule
symptoms
pain
frequency
1. Introduction
It is a rare form of endometriosis with a frequency of around 0.4–4% of extragenital lesions [1][2] and around 0.5–1% of all cases of endometriosis [3][4][5], and it has been reported that it represents 30–40% of cases of abdominal wall endometriosis (AWE) [6]. Generally, UE presents with a red, purple, or black umbilical nodule with a diameter ranging from 0.5 to 3 cm [7]. According to Hirata et al. (2020), the risk of malignant transformation of UE is about 3% [2].
Two types of umbilical endometriosis(UE) have been described. Primary UE occurs in the absence of a surgical history. Severe theories have been proposed, such as the migration of endometrial cells through the abdominal cavity, the lymphatic system, or embryonic remnants in the umbilical fold (e.g., the urachus and umbilical vessels); genetic predisposition; and immunologic defects [7]. Romera-Barba et al., demonstrated that the disease occurs after prolonged exposure to the metaplastic and environmental factors [8], whereas secondary UE arises on scar tissue following abdominal procedures such as laparoscopy [2][7]. The distinction between primary and secondary development of UE appears to be important for the understanding of the pathogenic mechanism of this disease form [9].
Hirata et al. [10] suggested guidelines for management of extragenital endometriosis and claims for umbilical endometriosis that radical surgery with wide local excision represents the primary treatment. Surgical excision is strongly recommended but with weak supporting evidence because of unknown long-term efficacy and complications. In contrast, medical treatment is weakly recommended due to limited supporting data and lack of studies comparing medical and surgical treatment for umbilical endometriosis.
2. Umbilical Endometriosis and Pathogenic Theory Proposal
The results of this analysis show that UE represents about 21% of all the abdominal wall(AWE) cases: Most lesions were primary UE (about 70%); a history of endometriosis was reported in almost one third of women of UE; pelvic lesions co-existed in about 35% of the patients; and pain and catamenial symptoms were the most commonly reported complaints.
Before discussing the results of this analysis, potential limitations should be considered. The selected studies reported large differences in the number of UE cases, with less than 10 cases included in most of the studies. However, effect of random fluctuation aside, the results of this analysis are largely consistent among different studies, particularly among the largest ones.
Although, when planning the systematic literature search, articles published in three languages were potentially considered, eventually the selected papers were almost all published in English, and only two in French [11][12] and one in Italian [13]. Authors may be more prone to publish in an international, English language journal if results are “newer”. Further, studies published before the year 1950 were excluded, as papers were sparse, frequently lacking histological diagnosis, and differed in diagnostic criteria.
The results of this systematic review are consistent with the findings of previous literature reviews. For example, in a review published in 2007 and including 122 patients with documented UE from 1966 to 2007 and 109 cases reported before 1953, the mean age of the study population was 37.7 years, and 27% of cases had a history of endometriosis [1].
Concerning symptoms, Calagna et al. [14] showed that intermittent pain in the umbilical area was the most common complaint reported by 66.0% of the study women, whereas cyclic bleeding from the umbilical site was described by 43.3% of them. The researchers findings are consistent but slightly higher than their results.
The data emerging from the researchers review show that the post-operative recurrence rate of the UE is very low. In fact, a total of 7/148 (4.7%, 95%CI 2.3–9.4) recurrences were observed during a follow-up period ranging from three to 92.5 months. This suggests that surgery is an effective treatment for this endometriotic lesion type.
An interesting finding of the present review is the fact that primary UE was the most common form; secondary was the iatrogenic form, representing 31.6% of cases. A history of endometriosis was reported by 37.9% of women. In a literature review published in 2015 (Calagna et al.) [14], the authors estimated that about 20% of cases of primary UE had a diagnosis of pelvic endometriosis. In the present review, this figure is substantially higher, as concomitant endometriotic lesions were observed in over one third of women who underwent pelvic visualization. This observation offers some insights in the pathogenesis of UE. Primary endometriosis is obviously the disease form of interest here, as the origin of secondary endometriosis is mainly iatrogenic.
In cases of isolated umbilical endometriosis, the disease might arise from metaplastic changes of urachal remnants [15]. Otherwise, in consideration of the frequency of UE among cases of AWE (21% in the researchers review), it can be hypothesized that endometrial cells may stem from the umbilical cord at birth. According to Calagna et al. (2015) [14], inflammation of the tissues around endometriotic pelvic implant may favor the shedding of endometriotic cells which may be transported through the venous vessels to the umbilicus.
However, when primary UE is associated with endometriosis at other, mainly pelvic, sites, the metastatic hypothesis appears also plausible. Of note, the prevalence of endometriotic pelvic lesions co-existent with primary UE was much higher than the usual estimates observed in the general premenopausal population [16][17][18][19]. This finding supports the possibility of a common etiological mechanism for the two disease locations because, if a separate pathogenesis exists for UE, the unusually high frequency of concomitant pelvic lesions would be difficult to explain.
If transtubal menstrual reflux is the origin of endometriosis, once in the pelvis endometrial cells might reach the umbilicus and implant directly on its parietal peritoneal surface without necessarily entering the vascular or lymphatic vessels. Indeed, a large body of evidence supports the notion that intra-abdominal endometriotic lesion distribution is determined to a large extent by physiological and anatomical factors that favor endometrial cell implantation [20].
Large bowel peristalsis combined with diaphragmatic respiratory movements, i.e., a physiologic factor, originate hydrostatic pressure variations that convey the peritoneal fluid from the pelvis along the right peritoneal gutter to the retro-hepatic and sub-phrenic area [21][22][23]. The falciform ligament, i.e., an anatomical factor, hampers the transit across the midline from the right to the left sub-phrenic space [24][25]. This explains the much higher prevalence of right-sided diaphragmatic, hepatic, and pleural endometriosis lesions compared with left-sided ones and could also elucidate the origin of UE.
In fact, once stuck by the falciform ligament, the peritoneal fluid containing free-floating endometriotic glands could be funneled by this crescentic peritoneal fold and by the hepatic round ligament toward the intra-abdominal aspect of the umbilicus, where endometriotic cells may eventually implant and infiltrate the overlying connective tissue. The presence of a small peritoneal concavity creates a sort of niche that would further facilitate endometrial cells implantation. This is also supported by reports of UE in women affected by umbilical hernia [26][27][28].
Even in the hypothesis of peritoneal fluid ascending directly from the pelvis along the anterior abdominal wall, endometrial cells would be channeled by the peritoneal folds created by the medial umbilical ligaments (obliterated umbilical arteries) and the median umbilical ligament (obliterated uracus), which create two anatomic preferential routes converging toward the umbilicus [29].
The pattern of dissemination of ovarian epithelial cancer cells might constitute a similar pathogenic model here. Both hematogenous and lymphatic spread have been suggested as the physio-pathological mechanism to explain umbilical metastases, the so-called Sister Mary Joseph’s nodules, from gynecologic cancers [3][30]. However, according to Hugen et al. (2021) [31], a local direct extension of cancer cells from the peritoneal aspect to the skin is possible, as the umbilicus is a deep structure directly connected to the extraperitoneal tissue.
Indeed, a contiguous extension of ovarian cancer cells from the peritoneal surface to the umbilical skin has been demonstrated [32]. Moreover, in a recent nationwide review of pathology records of umbilical metastases diagnosed in the Netherlands between 1979 and 2015, almost three fourths of the 806 study patients were females and, in these cases, umbilical metastases most frequently originated from the ovaries [31].
The peritoneal fluid conveyance hypothesis would be in line with evidence on endometriosis affecting the liver, diaphragm, and pleura [20][33][34], and would add further support to the unifying mechanistic theory of endometriosis as a disease originating from menstrual reflux and subsequent abdominopelvic distribution of lesions according to specific local characteristics that facilitates implantation [35]. If this is the case, primary UE should be considered part of the “right hypochondrium complex” [20]. Of note, this hypothesis would explain primary UE even in the absence of co-existent pelvic implants, as the origin of ectopic umbilical endometriotic glands would be the retrograde menstrual flow per se, independently of already extant pelvic localizations of the disease.
The sine qua non for the validity of this theory is the demonstration of a continuum of endometriotic infiltration through the entire thickness of the umbilical scar with involvement of the parietal peritoneal layer, as shown in Figure 1, Figure 2 and Figure 3.
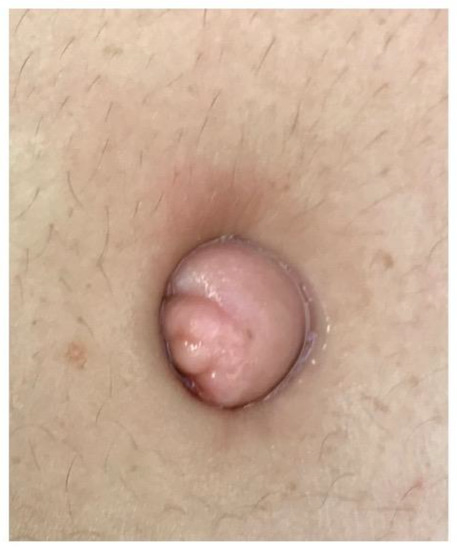
Figure 1. Endometriosis infiltrating the entire skin aspect of the umbilicus.
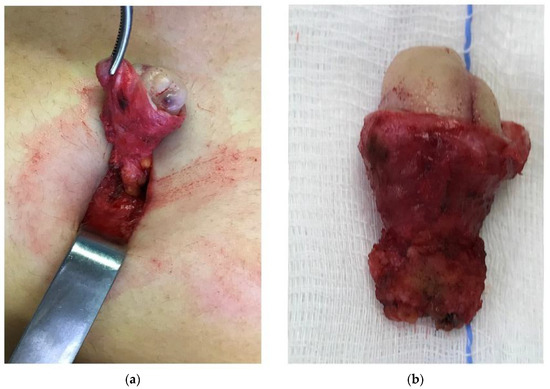
Figure 2. The umbilical peduncle is dissected down to the parietal peritoneum, which is included in the resected tissue (a). Anatomical specimen of en-bloc resection of umbilical endometriosis from the skin aspect to the parietal peritoneum (b).
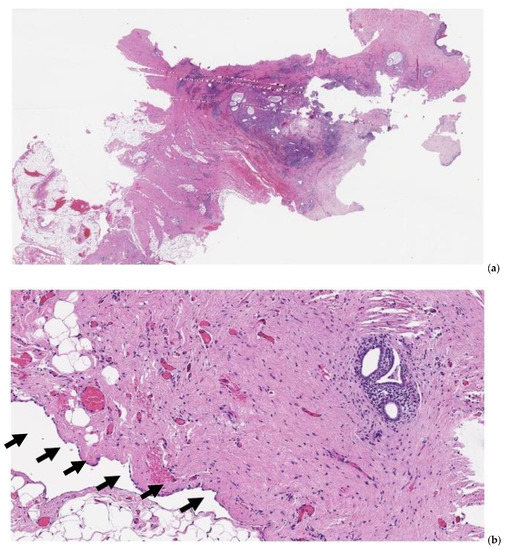
Figure 3. Pathology specimen after full-thickness omphalectomy for umbilical endometriosis. At scanning magnification ((a), hematoxylin-eosin, 10×), foci of endometriosis are apparent, spanning from the deeper tissues of the abdominal wall throughout the dermal layer (left to right). At higher magnification ((b), hematoxylin-eosin, 100×), endometrial glands are identified in the stroma underlying the parietal peritoneal mesothelial surface (arrows).
Future studies on UE should focus on detailed histopathology findings of those women who undergo en-bloc omphalectomy. In addition, when laparoscopy is also indicated, a careful visual inspection of the diaphragm after liver retraction by means of a blunt probe should be performed to identify concomitant endometriotic implants [36]. These measures would both help clarify whether UE originates from inside the abdominal cavity or is a purely skin condition with a separate pathogenesis.
References
- Victory, R.; Diamond, M.P.; Johns, D.A. Villar’s nodule: A case report and systematic literature review of endometriosis externa of the umbilicus. J. Minim. Invasive Gynecol. 2007, 14, 23–32.
- Hirata, T.; Koga, K.; Kitade, M.; Fukuda, S.; Neriishi, K.; Taniguchi, F.; Honda, R.; Takazawa, N.; Tanaka, T.; Kurihara, M.; et al. A national Survey of Umbilical Endometriosis in Japan. J. Minim. Invasive Gynecol. 2020, 27, 80–87.
- Dubreuil, A.; Dompmartin, A.; Barjot, P.; Louvet, S.; Leroy, D. Umbilical metastasis or Sister Mary Joseph’s nodule. Int. J. Dermatol. 1998, 37, 7–13.
- Markham, S.M.; Carpenter, S.E.; Rock, J.A. Extrapelvic endometriosis. Obstet. Gynecol. Clin. N. Am. 1989, 16, 193–219.
- Michowitz, M.; Baratz, M.; Stavorovsky, M. Endometriosis of the umbilicus. Dermatologica 1983, 167, 326–330.
- Kyamidis, K.; Lora, V.; Kanitakis, J. Spontaneous cutaneous umbilical endometriosis: Report of a new case with immunohistochemical study and literature review. Dermatol. Online J. 2011, 17, 5.
- Chamié, L.P.; Ferreira, R.R.D.M.; Tiferes, D.A.; de Macedo, N.A.C.; Serafini, P.C. Atypical sites of deeply infiltrative endometriosis: Clinical characteristics and imaging findings. Radiographics 2018, 38, 309–328.
- Romera-Barba, E.; Ramón-Llíon, J.C.; Pérez, A.S.; Navarro, G.I.; Rueda-Pérez, J.M.; Maldonado, A.J.C.; Vazquez-Rojas, L. Endometriosis umbilical primaria. A propósito de 6 casos. Rev. Hispanoam Hernia 2014, 2, 105–110.
- Mechsner, S.; Bartley, J.; Infanger, M.; Loddenkemper, C.; Herbel, J.; Ebert, A.D. Clinical management and immunohistochemical analysis of umbilical endometriosis. Arch. Gynecol. Obstet. 2009, 280, 23542.
- Hirata, T.; Koga, K.; Kai, K.; Katabuchi, H.; Kitade, M.; Kitawaki, J.; Kurihara, M.; Takazawa, N.; Tanaka, T.; Taniguchi, F.; et al. Clinical practice guidelines for the treatment of extragenital endometriosis in Japan, 2018. J. Obstet. Gynaecol. Res. 2020, 46, 2474–2487.
- Abramowicz, S.; Pura, I.; Vassilieff, M.; Auber, M.; Ness, J.; Denis, M.H.; Marpeau, L.; Roman, H.J. Umbilical endometriosis in women free of abdominal surgical antecedents. Gynecol. Obstet. Biol. Reprod. 2011, 40, 572–576.
- Darouichi, M. Primary and secondary umbilical endometriosis. Feuill. Radiol. 2013, 53, 21–26.
- Lattuneddu, A.; Ercolanni, G.; Orlandi, V.; Saragoni, L.; Garcea, D. Umbilical endometriosis: Description of 3 cases and diagnostic and therapeutic observations. Chirugia. Dec. 2002, 15, 209–211.
- Calagna, G.; Perino, A.; Chianetta, D.; Vinti, D.; Triolo, M.M.; Rimi, C.; Cucinella, G.; Agrusa, A. Primary umbilical endometrioma: Analyzing the pathogenesis of endometriosis from an unusual localization. Taiwan J. Obstet. Gynecol. 2015, 54, 306–312.
- Mizutani, T.; Sakamoto, Y.; Ochiai, H.; Maeshima, A. Umbilical endometriosis with urachal remnant. Arch. Dermatol. 2012, 148, 1331.e2.
- Rowlands, I.J.; Abbott, J.A.; Montgomery, G.W.; Hockey, R.; Rogers, P.; Mishra, G.D. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 657–665.
- Christ, J.P.; Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Reed, S.D. Incidence, prevalence, and trends in endometriosis diagnosis: A United States population-based study from 2006 to 2015. Am. J. Obstet. Gynecol. 2021, 225, 500.e1–500.e9.
- Eisenberg, V.H.; Weil, C.; Chodick, G.; Shalev, V. Epidemiology of endometriosis: A large population-based database study from a healthcare provider with 2 million members. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 55–62.
- Gylfason, J.T.; Kristjansson, K.A.; Sverrisdottir, G.; Jonsdottir, K.; Rafnsson, V.; Geirsson, R.T. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am. J. Epidemiol. 2010, 172, 237–243.
- Vercellini, P.; Abbiati, A.; Viganò, P.; Somigliana, E.; Daguati, R.; Meroni, F.; Crosignani, P.G. Asymmetry in distribution of diaphragmatic endometriotic lesions: Evidence in favour of the menstrual reflux theory. Hum. Reprod. 2007, 22, 2359–2367.
- Foster, D.C.; Stern, J.L.; Buscema, J.; Rock, A.; Woodruff, J.D. Pleural and pulmonary endometriosis. Obstet. Gynecol. 1981, 58, 552–556.
- Rosenshein, N.B.; Leichner, P.K.; Vogelsang, G. Radiocolloids in the treatment of ovarian cancer. Obstet. Gynecol. Surv. 1979, 34, 708–720.
- Drye, J.C. Intraperitoneal pressure in the human. Surg. Gynecol. Obstet. 1948, 87, 472–475.
- Meyers, M.A. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1973, 119, 198–206.
- Meyers, M.A. The spread and localization of acute intraperitoneal effusions. Radiology 1970, 95, 547–554.
- Leite, G.K.; Carvalho, L.F.; Korkes, H.; Guazzelli, T.F.; Kenj, G.; Viana Ade, T. Scar endometrioma following obstetric surgical incisions: Retrospective study on 33 cases and review of the literature. Sao Paulo Med. J. 2009, 127, 270–277.
- Saito, A.; Koga, K.; Osuga, Y.; Harada, M.; Takemura, Y.; Yoshimura, K.; Yano, T.; Kozuma, S. Individualized management of umbilical endometriosis: A report of seven cases. Obstet. Gynaecol. Res. 2014, 40, 40–45.
- Yuen, J.S.; Chow, P.K.; Koong, H.N.; Ho, J.M.; Girija, R. Unusual sites (thorax and umbilical hernial sac) of endometriosis. J. R. Coll. Surg. Edinb. 2001, 46, 313–315.
- Hegazy, A.A. Anatomy and embryology of umbilicus in newborns: A review and clinical correlations. Front. Med. 2016, 10, 271–277.
- Gabriele, R.; Conte, M.; Egidi, F.; Pietrasanta, D.; Borghese, M. Umbilical metastases: Current viewpoint. World J. Surg. Oncol. 2005, 3, 13.
- Hugen, N.; Kanne, H.; Simmer, F.; van de Water, C.; Voorham, Q.J.; Ho, V.K.; Lemmens, V.E.; Simons, M.; Nagtegaal, I.D. Umbilical metastases: Real-world data shows abysmal outcome. Int. J. Cancer 2021, 149, 1266–1273.
- Goodheart, R.S.; Cooke, C.T.; Tan, E.; Matz, L.R. Sister Mary Joseph’s nodule. Med. J. Aust. 1986, 145, 477–478.
- Prodromidou, A.; Machairas, N.; Paspala, A.; Hasemaki, N.; Sotiropoulos, G.C. Diagnosis, surgical treatment and postoperative outcomes of hepatic endometriosis: A systematic review. Ann. Hepatol. 2020, 19, 17–23.
- Nezhat, C.; Lindheim, S.R.; Backhus, L.; Vu, M.; Vang, N.; Nezhat, A.; Nezhat, C. Thoracic endometriosis syndrome: A review of diagnosis and management. JSLS J. Soc. Laparoendosc. Surg. 2019, 23, e2019.00029.
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275.
- Nezhat, C.; Seidman, D.S.; Nezhat, F.; Nezhat, C. Laparoscopic surgical. Management of diaphragmatic endometriosis. Fertil. Steril. 1998, 69, 1048–1055.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
623
Revisions:
2 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




