Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisa Elviri | + 7502 word(s) | 7502 | 2022-02-18 05:13:15 | | | |
| 2 | Amina Yu | -144 word(s) | 7358 | 2022-02-18 10:55:48 | | | | |
| 3 | Amina Yu | -175 word(s) | 7327 | 2022-02-18 11:01:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Elviri, L. 3D Printing Technologies in Biosensors Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/19620 (accessed on 07 February 2026).
Elviri L. 3D Printing Technologies in Biosensors Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/19620. Accessed February 07, 2026.
Elviri, Lisa. "3D Printing Technologies in Biosensors Production" Encyclopedia, https://encyclopedia.pub/entry/19620 (accessed February 07, 2026).
Elviri, L. (2022, February 18). 3D Printing Technologies in Biosensors Production. In Encyclopedia. https://encyclopedia.pub/entry/19620
Elviri, Lisa. "3D Printing Technologies in Biosensors Production." Encyclopedia. Web. 18 February, 2022.
Copy Citation
Three-dimensional (3D) printing was born in 1986, with the publication of Chuck Hull’s patent, who invented stereolithography; it has evolved and differentiated, with the introduction of new printing techniques and numerous materials with different characteristics.
3D printing technologies
biosensors
electrochemical biosensors
material extrusion
vat photopolymerization
material jetting
1. 3D Printing and Biosensors Production
3D printing technologies offer promising innovation in the manufacturing of biosensors or biosensor components. The most relevant scientific literature dealing with this topic is reported in Table 1. Table 1 provides a summary of the 3D printing technologies used to fabricate biosensors or the parts of a biosensor, the material used for printing, the application field, and the analytical applications. It was grouped publications on the basis of the 3D printing technology used in the biosensor preparation and we discuss them as a function of the application field. The application fields, the principal analytical purposes, and the limits of detection of the analytes investigated in different biological matrices are reported. The main 3D printing technologies applied in the works considered here are material extrusion (ME), vat photopolymerization (VP), and material jetting (MJ); ME consists principally of fused deposition modelling (FDM), inkjet printing, and aerosol-jet-printed (AJP) methods; VP is based primarily on stereolithography (SLA), while MJ utilizes MultiJet technology. Lastly, some examples of other 3D printing technologies for biosensors production such as powder bed fusion and binder jetting, as well as some combined approaches, are listed.
Table 1. 3D printing technologies in biosensors production, their fields of application, and the main analytical purposes. Limits of detection and biological sample of interest are also reported when the information was available in related publications (N/A = not available).
| 3D Printing Technologies | Field of Application | Analytical Purposes | Limit of Detection | Biological Sample | 3D-Printed Materials | Ref. |
|---|---|---|---|---|---|---|
| Material Extrusion: FDM | Biomedical | Hydrogen peroxide detection | 11.1 µM | N/A | Graphene/PLA | [1] |
| Material Extrusion: FDM | Biomedical: wearable sensors | Glucose determination in human sweat | 1.2 µmol/L | Human sweat | Carbon PLA/TPU | [2] |
| Material Extrusion: FDM | Biochemical: chiral sensors | Tryptophan enantiomers resolution and quantification | N/A | N/A | PLA | [3] |
| Material Extrusion: FDM | Biomedical: diagnosis | Anticancer drugs direct quantification | 5 × 10−8 M in serum | Human biological fluids | PLA | [4] |
| Material Extrusion: FDM | Biomedical: point-of-care (POC) diagnostics | DNA amplification (LAMP) | N/A | Human saliva | PP | [5] |
| Material Extrusion: Inkjet printing | Biomedical | Epithelial cell cultures monitoring | 4.36 cell-index unit/cells × cm−2 | Epithelial cells | AgNPs/SU-8 | [6] |
| Material Extrusion: AJP | Biomedical | Cytokine monitoring in bovine serum | IFN-γ: 25 pg/mL; IL-10: 46 pg/mL | Bovine serum | Graphene-nitrocellulose | [7] |
| Material Extrusion: AJP | Biomedical: diagnosis | SARS-CoV-2 antigens detection | S1 protein: 2.8 × 10−15 M; RBD: 16.9 × 10−15 M | Human biological fluids | AuNPs-PDMS | [8] |
| Material Extrusion: FDM | Point-of-care diagnostics | Dopamine detection | 1.45 µg/mL | N/A | CNT/CB/PLA | [9] |
| Material Extrusion: FDM | Biomedical: epithelial cancer biomarkers detection | Mucin 1 quantification | 80 nM | Breast cancer cells | Nanocarbon-PLA | [10] |
| Material Extrusion: DLP | Biomedical: multiplexed protein biomarker ELISA | IL-6, CRP, CEA, PSA | IL-6: 1.75 pg/mL; CRP: 26 pg/mL; CEA: 7.5 pg/mL; PSA: 62 pg/mL | Rat Plasma | PEDGA | [11] |
| Material Extrusion: FDM | Biochemical and Biophysical | Protein absorption | N/A | N/A | PLA | [12] |
| Material Extrusion: FDM | General: miniaturized electrochemical sensor systems | Cyclic voltammetry of redox couple standard and potentiometric pH measurements | N/A | N/A | ABS | [13] |
| Material Extrusion: inkjet printing-drop-on-demand printer | Biosensors manufacturing optimization | Catalytic activity and conformational changes evaluation in enzymes | N/A | N/A | PP | [14] |
| Material Extrusion: inkjet printing-drop-on-demand printer | Biocompatible conductive ink fabrication for neuronal sensing | Graphene patterns-based conductive inks | N/A | N/A | Graphene-PI | [15] |
| Material Extrusion | Food and feed quality | Mycotoxins quantification | DON: 0.07; 3-ADON: 0.10; 15-ADON: 0.06 μg/mL | Food and feed | Gelatin-Methacryloyl | [16] |
| Material Extrusion: FDM | Environmental: water pollution monitoring | Herbicides (atrazine and acetochlor) detection | Atrazine: 0.24 ppb; acetochlor: 3.2 ppb | Water | PLA | [17] |
| Material Extrusion: FDM | Environmental (water pollution monitoring) and Biomedical | Serotonin quantification in synthetic urine and catechol determination in water | Serotonin: 0.032 μmol/L; catechol: 0.26 μmol/L | Synthetic biological fluids and water | Graphene oxide-PLA | [18] |
| Material Extrusion: FDM | Quality control: biofuels | Copper determination in bioethanol | 0.097 μg/L | Biofuels | Carbon black-PLA | [19] |
| Material Extrusion: inkjet printing-direct ink writing | General: battery safety | Gas detection in lithium-ion batteries | N/A | Li-ion batteries | CuMPs-polyethylene oxide | [20] |
| Vat Photopolymerization | Biomedical: living biosensor | In situ monitoring of cellular metabolites | N/A | Cells | Au.pHEMA | [21] |
| Vat Photopolymerization | Biomedical: point-of-care (POC) diagnostics | Tumor markers (alpha-fetoprotein) detection | 0.01 ng/mL | Human blood | N/A | [22] |
| Vat Photopolymerization | Biomedical: diabetics diagnosis | Glucose determination in human sweat and blood | 25 μM | Human sweat and blood | rGO-TEPA/PB | [23] |
| Vat Photopolymerization | Biomedical | Glucose and cholesterol quantification in human blood | Glucose: 1.2 μM; cholesterol: 2.3 μM | Human blood | White resin | [24] |
| Vat Photopolymerization: SLA | Biomedical: living biosensor | Stereolithographic printing of engineered microbial in biosensor for in situ monitoring of uranium in groundwater | 2.5 μM | Groundwater | PEGDa | [25] |
| Vat Photopolymerization: SLA | Biomedical | Metastatic cancer biomarkers quantification | DSG3: 0.10 fg/mL; VEGF-A, VEGF-C, β-Tub: 0.20 fg/mL | Human biological fluids | Chitosan | [26] |
| Vat Photopolymerization: SLA | Biomedical: biocompatible biosensor | Biocompatibility evaluation of commercial resins towards rat cardiomyocytes | N/A | Rat cardiomyocytes | Commercial resins | [27] |
| Vat Photopolymerization: digital light processing | Biomedical: cancer diagnosis | Circulating tumor cells (CTCs) detection in human blood | 10 cells/mL | Human blood | PU | [28] |
| Vat Photopolymerization: digital light processing | Biomedical: biomarker detection in complex matrices | C-reactive protein as model biomarker | 1 ng/mL | Fetal bovine serum | Plastic material (Not specified) | [29] |
| Vat Photopolymerization | Quality control: foods | Salmonella typhimurium detection in food | 17 CFU/mL | Food | ABS | [30] |
| Vat Photopolymerization: SLA | Quality control: agri-food matrices | Antioxidant capacity (TAC) in food extracts and beverages | Gallic acid equivalent: 30 μM | Food and beverages | N/A | [31] |
| Vat Photopolymerization: SLA | Biophotonic technologies | 3D-printed transfer molding for photonic biosensor optimization | N/A | N/A | PAMPSA-PAAm | [32] |
| Vat Photopolymerization: SLA | Wearable and implantable bioelectronics, robotics, energy storage, and cell cultures | Logic of architecture design applied to conductive hydrogel manufacturing | N/A | N/A | N/A | [33] |
| Material Jetting: MultiJet technology | Biomedical: early diagnostics | Cancer metastasis monitoring | 106 cells/mL | Human biological fluids | VisiJet M3 crystal | [34] |
| Material Jetting: MultiJet technology | Biomedical: portable-living biosensor | Cell-based biosensor for volatile compounds detection | 1-octen-3-ol: 1 µM | N/A | VisiJet M2R-CL | [35] |
| Material Jetting: MultiJet technology | Biomedical | Proteins detection | 0.04 µM | Human biological fluids | VisiJet M2R-CL | [36] |
| Material Jetting: MultiJet technology | Biomedical: aptamer-based impedimetric biosensor | Escherichia coli label-free detection | 105 cells/mL | Fecal material | PMMA | [37] |
| Material Jetting: fluid dynamic modelling | Environmental: water pollution evaluation | Freshwater toxicity monitoring | Ni(II), Cr(III): < 2 mg/L | Freshwater | N/A | [38] |
| Binder Jetting | Biomedical: allergy diagnosis | Immunoglobulin E detection | 0.2 µg/mL | Human blood | PMMA | [39] |
| Powder Bed Fusion | Biomedical: point-of-care (POC) diagnostics-wearable biosensor | Real-time monitoring of electrical body signals | N/A | N/A | Sugar grains | [40] |
| 3D-printed modular magnetic digital microfluidic: SLA + FDM | Biomedical: point-of-care (POC) diagnostics | Biomarkers sensing, pathogen identification, antibiotic resistance determination, glucose and protein quantification | HBsAg: 61.6 ng/mL; CRP: 59.8 ng/mL; BSA: 54.6 µg/mL; glucose: 0.47 mg/dL | Human biological fluids | Clear resins/ABS | [41] |
| Inkjet printing + Microlithography | Bioelectronics | Quantitative comparison between microlithography and 3D printing in hydrogels manufacturing for biosensing and tissue engineering | N/A | N/A | PEDOT.PSS/p(HEMA-co-EGMA) | [42] |
2. Material Extrusion and Biosensors
Material extrusion 3D printing is one of the most common printing methodologies due to its simplicity of use, wide applicability, and precision. 3D printers based on an extrusion system use a screw device or a pneumatic actuator to deliver the ink through a needle or a nozzle for material deposition for the object creation. These common extrusion methods are compatible with thermoplastic materials such as polylactic acid (PLA) or acrylonitrile butadiene styrene (ABS), PC (polycarbonate), PP (polypropylene) and so on . Material deposition in X, Y, and Z axes are controlled by actuators that regulate the orientation of the nozzle in three dimensions, and each layer is built on top of the previous [43][44], tracing the printed object dimension and their design specified in the standard triangle/ file (STL), a file format extensively used for 3D printing and rapid prototyping. Amongst the 3D printing approaches based on material extrusion technology, fused deposition modelling (FDM) also known as fused filament fabrication (FFF) represents one of the most employed thanks to its ability to create complex geometries, its affordability, and simplicity to use [3][13]. In FDM, a polymer or polymer mixture, melted by a heated cartridge or a print head, is pushed through a syringe to create structures with a well-defined architecture [44]. However, this approach has some drawbacks, as the printing process is slow compared to other techniques such as stereolithography (SLA); furthermore, the quality of the printing is adequate but lower in comparison with the overall precision obtained from binder jetting and vat photopolymerization printing methods. Despite these limitations, FDM is perfectly suitable for rapid prototyping. Therefore, it is used for a wide variety of purposes, from digital healthcare and pharmaceutical applications [45] to automotive and aerospace sectors [46]. Another example of 3D ME technology is inkjet printing—a versatile technique in which electrical actuators are used to eject pico-liter volumes of liquid from micron-sized nozzles onto a substrate in a defined pattern with a layer-by-layer process. This technique is easily adaptable to a wide range of liquid materials or solid suspensions, from conductive polymers and dielectric inks to proteins and living cells with no post-processing required. Inkjet technology is efficiently exploited for the printing of paper documents, but applications in different fields such as organic electronics, sensor fabrication, chemical synthesis, and biology are reported [6][47][48]. A promising 3D technology is related to aerosol jet printing (AJP) for digital additive manufacturing at the microscale level (range from 10 μm to 100 nm). It is a direct-write additive manufacturing technique allowing for the printing of patterned circuits with high spatial resolution, avoiding the need for chemical etching. Through the use of different inks, including functional nanomaterial, AJP can be applied on different substrates, including conductors, dielectrics, semiconductors, and polymers. In some cases, consistency and reproducibility are challenging to achieve. Despite these limitations, AJP is adaptable on both 2D and 3D substrates [7][8][49]. Thus, in the last two years, all these ME 3D printing technologies have been used for biosensors or, at least, some of their components have been fabricated with applications in electrochemistry studies, from bioelectronics applied to medical purposes [50] to environmental monitoring [51] and food safety assessment [52].
2.1. Material Extrusion for Biomedical Applications
Material extrusion, due to its simplicity of use and general low-cost of employment, allows the rapid prototyping of different apparatuses that can be exploited in different fields. One of the most reported in the literature is represented by biomedical applications. This paragraph gathers different examples on this topic, starting from the development of electrochemical systems suitable for healthcare monitoring. Most of electrochemical biosensors are manufactured manually; this approach inevitably leads human error. The utilization of 3D printing technologies helps to overcome these limitations, improving the properties of biosensors in terms of the conductivity and analytical performance of the system, allowing a high-throughput detection. Thus, regarding electrochemical measurements, 3D printing technology has been used to fabricate customized 3D-printed electrodes as a platform on which to develop biosensing, energy generation, and storage devices.
Marzo et al. [1] demonstrated a very interesting and innovative 3D-printed enzymatic graphene–polylactic (PLA) electrode, developed by material extrusion technology, for direct electron transfer using horseradish peroxidase enzyme for hydrogen peroxide detection. In order to confirm and facilitate heterogeneous electron transfer, gold nanoparticles were included in the system. The experimental data reported an analytical linear range of hydrogen peroxide concentration equal to 25–100 µM, and the 3D-printed electrode showed a limit of detection of 11.1 µM and a limit of quantification of 37 µM. This work creates an innovative perspective for the manufacture of third-generation electrochemical biosensors using 3D printing technology. Indeed, the utilization of graphene in 3D printing is gaining interest due to the unique properties of this material, in terms of subtlety, flexibility, and mechanical strength. Biosensors produced in this way are perfectly suitable for applications in biomedical fields (e.g., detect glucose and other biomarkers in biological fluids without using electron mediators and binder polymers), according to the needs of tailorable devices with fast and cost-reducing manufacturing features [1].
A further example of material extrusion applied to point-of-care diagnostic devices development was reported by Katseli et al. [2]. FDM 3D printing was employed for the manufacture of an electrochemical ring (e-ring). The 3D-printed e-ring represented a wearable sensor for glucose index measured in sweat. This work was a well-conceived example of a portable POC diagnostic device, accessible in terms of costs and fabrication process. The 3D-printed components were designed using free online software, and the biosensor was thought to be accessible to a smartphone, which represents something that most people use in their daily life. The system was composed of an enduring and flexible cylindrical holder made of TPU (thermoplastic polyurethane) and three electrodes fabricated using conductive filaments, such as carbon-loaded PLA and ABS from different manufacturers. Interestingly, carbon-based PLA showed better sensitivity in glucose detection; for this reason, it was selected as material for the 3D printing of all the electrodes. An electrodeposited gold film was applied to modify the e-ring before coupling with a miniature potentiostat directly accessible by a smartphone. This device allowed for the noninvasive, nonenzymatic amperometric self-testing of glucose levels in human sweat at the concentration range of 12.5−400 μmol L−1. An advantage is the absence of interference from common electroactive metabolites. The device was tested for its within-sensor reproducibility (3.4%, n = 6) and its long-term and mechanical stability, demonstrating solid results. The reproducibility of the sensors was calculated in terms of relative standard deviation (expressed in percentage) from measurements performed by four different devices—in this case, the value obtained was 6.8%. Both of these results demonstrated satisfactory repeatability in glucose detection and manufacturing process reproducibility. Wang et al. [3], thanks to FDM technology, overcame the crucial challenge of chiral recognition for electrochemical sensors with similar physicochemical properties such as enantiomers; in particular, the 3D-printed sensor was employed for the chiral recognition of L and D tryptophan (Trp) in a racemic solution. Fe3O4 nanoparticles were mixed with 1,3,5-tris(p-formylphenyl)benzene and 4,4″-diamino-p-terphenyl under acidic conditions to obtain a Fe3O4-magnetic covalent organic framework (COF); subsequently, they added Fe3O4@COF to bovine serum albumin (BSA) as chiral surface. Lastly, they functionalized the 3D-printed nanocarbon electrode with Fe3O4@COF@BSA to obtain an integrated 3D-printed nanocarbon electrode electrochemical chiral sensor, demonstrating, for the first time, the suitability of 3D printing technologies for this application. Linear sweep voltammetry was performed for the chiral recognition of the Trp isomers; the COF@BSA-functionalized 3D-printed electrode demonstrated higher efficiency in chiral recognition of L than D-Trp, and the system showed excellent repeatability for the relative quantification of the L-isomer in the racemic solution; reporting a good linearity (R2: 0.995) that allowed the chiral discrimination of the L-D enantiomers. It was showed taht potential for protein and porous material-modified 3D-printed electrodes in determining individual enantiomers in a mixture [3]. With reference to the diagnostics field, many detection technologies, such as gas chromatography or liquid chromatography coupled to mass spectrometry and enzymatic immunoassay are broadly applied to drug analysis. These analytical techniques can be very accurate and reliable, but they are time-consuming, they need sample pre-treatment, and furthermore they often require trained personnel to perform the analysis. These factors significantly limit their applications for drug analysis in clinical diagnosis [4]. To overcome these limitations, biosensors with specific features for clinical application have been developed as provided by Cheng et al. [4]. A 3D-printed portable paper cartridge was developed using FDM technology as a portable tool for the quantitation of drug in biological fluids, such as blood, urine, and saliva for application in clinical analysis. The 3D-printed cartridge consisted of a device cover, a sampler, and a device chassis . CAD software was used to design the structure of the object that was then manufactured by FDM 3D printer by using polylactic acid as material. The paper tip was finalized for samples preconcentration. By deposition of silver nanowires at the tip, the 3D-printed paper cartridge was used for sample pre-concentration and as a surface-enhanced Raman scattering (SERS) substrate to optimize the Raman signal for quantitative analysis. Each part was rationalized in a way such that the sampler was conceived for the slow and uniform release of sample solutions, the cover with grooves was fundamental for sampler stability, and the device chassis was structured in a way to improve sample pre-concentration of the paper tip. The procedure was performed in three main steps: the first step was adding sample into the 3D-printed sampler; the second step involved the transfer of the sample to the hydrophilic wick of the paper tip, which is where the pre-concentration of the sample started. The advantage of this device is that the preconcentration step occurs very quickly, with only few minutes being required, and after this period of time, the cartridge can be then removed and dried. A Raman spectroscopy was finally used to detect SERS from the cartridge. Following this procedure, each sample could be measured and quantified very rapidly. The pre-concentration capability of the cartridge significantly improved the fluorescence signal, allowing a 9.93-fold improvement in the overall SERS analysis. Compared with the multiple cooperation and multi-step methods seen in the existing technologies, this system represents a simple, cheap, and portable 3D-printed paper cartridge able to be integrated with a detection procedure within an hour. The performance of the above mentioned 3D-cartridge was evaluated by the quantitative detection of two broad spectrum anti-neoplastic drugs—epirubicin hydrochloride and cyclophosphamide—in bovine serum and artificial urine. It was presented a low-cost, portable, time-saving, 3D-printed device capable of performing the simultaneous determination of two different anticancer drugs widely used in chemotherapy, opening new perspectives on potential clinical applications [4].
Regarding the employment of fused deposition modelling (FDM) technology for the manufacture of point-of-care (POC) devices, Pantazis et al. [5] developed a 3D-printed bioreactor able to perform loop-mediated isothermal amplification (LAMP) on DNA collected from saliva samples to monitor the CYP2C19×2 mutation. This mutation is involved in the metabolism of the drug clopidogrel (Plavix), adopted for cardiovascular diseases treatment. The system showed significant advantages in terms of cost (less than EUR 30) and time of printing and assembly (2 h), demonstrating it to be perfectly adequate for the customized prototyping of point-of-care diagnostics. Furthermore, the ability to provide information about the safe and the effective use of a therapeutic drug to a specific person demonstrated the suitability of the device as a diagnostic system. 3D printing technologies could be exploited not only to build an ad hoc chassis and multiple elements for the device’s architecture but also to produce printable biological-based ink. In the quest for innovative biomaterials for advanced therapies, hydrogels represent a natural, biocompatible, and biodegradable solution. Hydrogel scaffolds are mainly composed of polysaccharides that can be obtained from renewable and recoverable natural sources, such as algae (e.g., alginate), animals (e.g., hyaluronic acid, chitosan, and chondroitin), plants (e.g., cellulose nanocrystals pectin, starch), and microorganisms (e.g., xanthan gum, pullulan, or dextran). The chemical, physical, and biological properties of these polymers ensure their high biocompatibility, activity, and reduced enzymatic degradability. Moreover, the presence of hydroxyl, carboxyl, amino, and other hydrophilic chemical groups allows for drug interaction and a series of multiple applications [53]. As a consequence, these active materials have been used for different applications, such as regenerative medicine, drug delivery, treatment of infected wounds, and eco-friendly water purification systems [53][54][55][56]. Moreover, printable biomaterials can interact with cells by physical and chemical binding at different levels, from individual cells up to a single molecule as a function of time and system dimension [57]. In this scenario, the investigation of the interactions between living cells and biomaterials could represent a valuable tool to better understand the mechanisms behind different molecular pathways of biology. Zanotti et al. [57] investigated the adaptation of lipid the profile of human fibroblasts to alginate 2D films and 3D-printed scaffolds. The 3D alginate scaffold was constructed using a homemade 3D printer. The alginate formulation (6% w/v) was printed by an extrusion process from a 26G needle in a layer-by-layer mode on the frozen printing plate (−14 °C). The 3D-printed scaffold was post-processed by immersion in the in a gelling solution of CaCl2 (3% w/v) or FeCl3 (3% w/v) for one hour. After rinsing with deionized water, the 3D scaffolds were ready to be tested on cell culture. Before evaluating the modulation of the relative expression of lipids in dermal human fibroblasts, Zanotti et al. performed a MTT colorimetric assay to monitor the viability of cells in contact with the biomaterial. The results proved the biocompatibility of these scaffolds with cells. Here, liquid chromatography–triple quadrupole tandem mass spectrometry was used for the selected determination of the lipidomic profile of fibroblasts grown on scaffolds. Targeted markers such as, ceramides (CER), lysophosphatidylcholines (LPC), free fatty acids (FFA), and lysophosphatidic acids (LPA) were analyzed. Except for the preparation procedure, the same protocols were followed with alginate 2D films. Targeted liquid chromatography–mass spectrometry analysis revealed that different scaffolds have the capabilities to affect the relative distribution profile of the main cell membrane lipids, which could result in a variation in membrane properties related to trafficking and signaling pathways. The behavior of human fibroblasts in contact with alginate hydrogels was demonstrated to be influenced by both architectures (2D and 3D). Intriguingly, 3D geometry can add an unknown physiologically relevant aspect compared with 2D [57]. Mainly in the biosensing field, but not only, the inkjet 3D printing techniques such as direct ink writing (DIW) and drop-on-demand (DOD), have also been widely employed for the fabrication of biosensors due to their advantages of contactless printing, reduction in waste, and rapid deposition [6][14][15][20]. Inkjet printing technology has been used by Mojena-Medina et al. [6] to fabricate interdigitated-electrode sensors (IDEs) to monitor epithelial cell cultures. In particular, the inkjet-printed sensor was used for monitoring the migration, proliferation, and detachment of a monolayer of keratinocytes (HaCaT) using real-time electrochemical impedance spectroscopy (EIS). IDEs have been constructed using flexible substrates based on polyethylene terephthalate (PET); silver nanoparticles (AgNPs) were added to provide a proper conductivity and also for their self-sintering property. Despite these credits, the cytocompatibility and the chemical stability of AgNPs are not well defined. Taking this consideration into account, to fabricate sensors able to perform impedance measurements, electrodes were isolated with dielectric-based ink (SU-8); SU-8 was chosen due to its properties as insulator but also as inert material, which represents a useful feature for exposure to cell lines. Inkjet 3D printing was chosen thanks to its maskless and contactless properties and its compatibility with current bioprinting techniques. IDEs were tested to perform impedance recordings on laboratory skin tissue. It have been found that variations in the impedance signal correlate linearly with cell density, reporting a sensitivity of 4.36 cell index units/cm2 with a linear regression between impedance variations and the initial value of cell density, with a coefficient of determination equal to 0.98. The relationship between impedance variations and cell status was further confirmed by fluorescence microscopy. Intriguingly, here the cell membrane was the main component affecting the total impedance. It was demonstrated that cell migration on the biosensor surface could be measured by impedance. The results obtained by monitoring this parameter were in agreement with those obtained by using the standard method based on image processing [6]. This work provides a valid alternative to monitor the in situ process associated with in vitro epidermal models for anchorage-dependent cells, skin substitutes, and tissue regeneration studies based on low-cost ink-jetted prototyping.
Finally, the use of 3D aerosol-jet-printing (AJP) technology in biosensors has been reported by Parate et al. [7], who developed an AJP graphene-based immunosensor for the simultaneous determination of two distinct cytokines in bovine serum: interferon gamma (IFN-γ) and interleukin 10 (IL-10). They selected graphene–nitrocellulose ink due to the high electrical conductivity of graphene at low thickness (nanometers scale). Using this 3D printing technology, they overcame the limit of traditional approaches used for graphene printing, such as inkjet printing. The latter technique is linked to ink viscosity requirement properties and issues related to high-resolution printed line width, which impedes the performance of graphene-printed biosensors by some printing techniques. It was optimized that the aerosol jet printing process steps, obtaining an unprecedented, with regard to graphene 3D-AJP, 40 µm width line resolution of the graphene–nitrocellulose-printed electrodes. This high resolution increased the signal-to-noise ratio reported in the electrochemical characterization and, consequently, improved the electrodes’ performance in cytokines detection, achieving wide sensing analytical range in serum—IFN-γ: 0.1–5 ng/mL and Il-10: 0.1–2 ng/mL; with high selectivity and low limits of detection: 25 pg/mL for IFN-γ and 46 pg/mL for IL-10. Interdigitated electrodes (IDEs) were annealed under CO2 conditions to introduce reactive oxygen species on the graphene surface that were able to covalently link IFN-γ and IL-10 antibodies functionalized to the graphene surfaces. Moreover, these biosensors showed optimal mechanical properties, especially in terms of flexibility. The results obtained reported that AJP-printed electrochemical immunosensors were suitable for monitoring cytokines in bovine serum with wide sensing range, low detection limit, and high selectivity without the need for sample prelabeling or preconcentration [7]. Graphene-based electrodes could also be used for the detection of pathogens involved in human disease onset and for food safety assessment. Another intriguing and virtually useful daily application of the AJP technique was proposed by Ali et al. [8]. It is a historical moment of emergency where there is a strong need for low-cost, portable, and reliable devices for the sensitive and rapid detection and early screening of disease biomarkers in the case of an outbreak. A prompt example was provided by coronavirus 2019 (COVID-19). A nanomaterial-based biosensing device was developed which is able to detect in few minutes through specific antibodies the SARS-CoV-2 spike S1 protein and its receptor binding domain. 3D nanoprinting was exploited in this work to design three-dimensional electrodes coated with nanoflakes of reduced graphene oxide in which the viral antigens were immobilized. The 3D-printed electrode was then integrated in a microfluidic device and assembled for use in a standard electrochemical cell. The antibodies were then distributed on the electrode surface, and when the viral antigens were selectively recognized, the variation in the impedance of the electrical circuit was detected. To record the signal variations a smartphone-based user-friendly interface was developed. A relevant technical aspect of the sensor is that it could be rapidly regenerated by introducing an acid solution that eluted the antibodies from the antigens, allowing subsequent analysis using the same platform. The device showed specific recognition of S1 and RBD detection; cross-reactivity and reproducibility studies were performed to assess this high sensitivity. Limits of detection reported were 2.8 × 10−15 M for the spike S1 protein and 16.9 × 10−15 M for its receptor binding domain (RBD). The explanation of this high-resolution detection capability could be attributed to the 3D architecture; the high porosity and the chemical properties of the surface’s platform allowed an enhanced loading capacity of the viral antigens. The proposed sensing system could also be useful in detecting biomarkers for other infectious agents, such as Ebola, HIV, and Zika, deepening the knowledge about immune response dynamics during infections [8].
2.2. Material Extrusion for Biophysical Studies, Electrochemical Measurements, and Enzyme-Based Ink Development
In addition to its widespread application in healthcare monitoring, where it demonstrated a remarkable applicability, material extrusion 3D printing has also been highlighted for its suitability in other fields of application, such as biophysics, with regard to the absorption properties of relevant molecules in biochemistry and molecular biology studies. In this regard, Samarentsis et al. [12] fabricated surface plasmon resonance (SPR) and love wave (LW) surface acoustic wave (SAW) sensors for biophysical and biochemical analysis. The final goal of this device was to facilitate simultaneous measurements of optical and acoustic signals for the study of biomolecules’ binding properties on a single surface. By using bovine serum albumin (BSA) as a protein model, two acoustic parameters, phase and amplitude of a LW, were carried out in synchronization with SPR readings. Figure 1 shows the experimental set-up assembly: in addition to the SPR/LW-SAW device, the system was equipped with a plastic holder combined with a polydimethylsiloxane (PDMS) microfluidic cell so that the platform could be used in flow-through mode [12]. By using a previously designed CAD object, 3D printing technology was used here to create a device holder in PLA for the electrical connection of the sensor device with the network analyzer. In order to have a valuable acoustic signal, the pressure applied on the system’s surface played a pivotal role. The specific holder incorporated miniaturized magnets, which allowed the application in each experiment of a standard pressure to the surface of the device by the flow cell. Six magnets, with diameter and thickness of 4 mm, were attached to the plastic holder by epoxy glue. The protein concentration estimated through real-time SPR measurements was reported as ΓSPR, with a value equal to 125 ± 13 ng/cm2. The developed system was the object of a systematic evaluation of optical and acoustic signals as a function of different surface perturbations, i.e., rigid mass loading (Au deposition), pure viscous loading (glycerol and sucrose solutions), and protein adsorption (BSA). The results obtained for this combined sensor set the fundamentals of future applications to other biochemical and biophysical studies, such as protein–protein and nucleic acid–protein interactions and the evaluation of surface topography influence on cell adhesion [12].
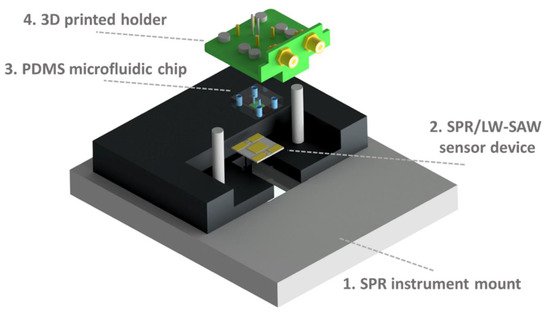
Figure 1. Experimental set-up assembly: First, the SPR/LW-SAW sensor device (2) is placed on the mount of the SPR instrument (1). Then, the PDMS chip (3) is fixed to the 3D-printed holder (4) and applied on the device surface. Reproduced with permission from Samarentsis et al. [12], Sensors, 20, 6177. Published by MDPI, 2020.
Deepening the possibilities offered by the FDM technology, in the wide range of diversified applications developed by this technique, it is possible to find 3D printing-based manufacturing process for the miniaturization of electrochemical devices. Miniaturization is gaining interest through the research community thanks to the increased willing to enhance the portability of diagnostic devices. This feature can, for example, allow the performance of in situ monitoring in places hard to reach with standard instrumentation. A practical example of that was reported by Sibug-Torres et al. [13]. It was developed and characterized a 3D-printed Ag|AgCl|gel-KCl reference electrode by fused deposition modelling that could be readily built on demand with low cost materials. These electrodes were integrated into 3D-printed miniaturized electrochemical sensor systems. The operations for fabricating the 3D-printed reference electrode (3D-RE) are illustrated in Figure 2a. The reference electrode was assembled by two main components—the casing and the junction—both manufactured using 3D printing; the other components were readily fixed into the assembled 3D-RE body. Since the 3D-RE was designed for application in aqueous samples, they selected acrylonitrile butadiene styrene (ABS) filament as printing material for its resistance to prolonged water exposure degradation. A cross section of the 3D-RE showing the internal architecture of the electrode is reported in Figure 2b, and a picture of assembled 3D-RE is also shown in Figure 2c. A 3D-RE without a junction installed (Figure 2(ci)) was taken to show the internal structure of the electrode, which included an Ag|AgCl wire and agar-KCl. In the above-mentioned work, 3D printing allowed the development of porous junctions that were able to limit the leakage of the chloride ion, thus maintaining a sufficient ion conduction between the internal electrolyte layer and the sample. 3D printer parameters, such as the filament extrusion ratio, can influence the junction porosity, and thus they were used to optimize the reference electrode’s potential stability and impedance. The 3D-RE developed was applied in cyclic voltammetry measurement of potassium ferricyanide and in pH sensing coupled with iridium oxide electrodeposited on a gold electrode [13]. The resulting 3D-RE was able to maintain a potential that was stable for at least 30 days under proper storage in 3M KCl. One of the most challenging parts of this work was the choice to exploit the porous property of the FFF-3D-printed materials, which is generally considered a defect, to improve the electrical properties of the reference electrode.
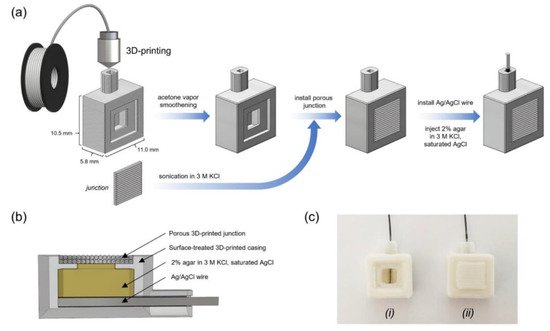
Figure 2. Design and fabrication of 3D-printed reference electrode (3D-RE). (a) Scheme illustrating the fabrication process of the 3D-RE. (b) Cross section of the 3D-RE. (c) Photograph of assembled 3D-RE (i) without a junction installed and (ii) with a junction installed. Reproduced with permission from Sibug-Torres et al. [13], Chemosensors, 8, 30. Published by MDPI, 2020.
An important issue that was just shallowly investigated is the overall effect of the printing process on active molecules, such as enzymes, that are added to bioink and deposited on different types of surfaces for the development of biosensors. One of the first reported studies about this intriguing topic was developed by Bai et al. [14]. A piezo-driven drop-on-demand (DOD) printer was used to investigate the effects of pressure wave propagation exerted by inkjet printing on enzyme activity and structural conformation. The wave superposition was measured, wave amplitude, resulting mechanical stress, and protein conformation change to compare the parallel printing of multiple enzymes having different sizes and structures. For the above-mentioned purpose, pyruvate oxidase, glucose oxidase, and peroxidase were employed as model enzymes. The catalytic activity of pyruvate oxidase was evaluated measuring the absorbance of quinonimine, produced from the oxidative reaction, at 550 nm. Interestingly, the mechanical stress increased the activity of pyruvate oxidase during the inkjet printing process. The mechanism behind this phenomenon was attributed to the mechanical activation or mild proteolysis that leads to variations in the three-dimensional conformation of the enzyme, improving its catalytic activity. Circular dichroism (CD) was performed on all three of the enzyme models to evaluate the eventual conformational change in the proteins during the bioprinting process; the proportion of secondary structures (α-elices and β-sheets) was more or less affected during the printing process, depending on the structural nature of the proteins. In this study, the pivotal role played by both the printing mechanism and the resulting structural and functional properties of the biomolecules involved on the final performance of the biosensor was demonstrated [14]. Asli et al. [15] presented a study where graphene was used as ink for a “drop-on-demand” 3D inkjet printer. This technology was employed in order to overcome the print instability issue relying on a time-saving process. Direct liquid phase exfoliation (DLPE) of graphite into graphene is attractive for inkjet printing, in particular for cell studies, since it was reported [58] that rat dopaminergic neuronal cells can adhere and live on graphene patterns. A scalable and aqueous phase exfoliation method of graphite was developed to obtain a high-quality graphene, which was employed as a tailored graphene ink with promising properties in terms of stability and electrical conductivity. The exfoliation process was based on the combination of bovine serum albumin (BSA) used as an exfoliating agent and a continuous low-speed wet-ball milling. The result of this interaction was the production of a water-dispersed graphene nanosheet. The main advantage of the printing system here described is the capability to significantly reduce graphene platelet disorientation in post-baked printed patterns with respect to commercially available inkjet printers. This means being able to minimize micro-scale junctions. It can be used as a reference guide and for further developments in the production of highly concentrated graphene and the patterning of circuits in the biosensor field [15].
2.3. Material Extrusion for Mycotoxins Analysis of Food and Feed
Mycotoxins are toxic compounds naturally synthesized by fungi. These secondary metabolites fit into the food chain consequent to an infection of crops, either before or after harvest, especially for cereals. When fungi find themselves to be under particular conditions in terms of humidity and temperature, they start to produce these toxic chemicals which, when undetected in food and feed, could represent a serious threat for human and animal health. To contain these harmful effects, a solid and reliable monitoring is required. In this scenario, Wei et al. [16] developed a screen-printed electrode constructed by extrusion-based 3D printing; the specific process (e.g., FDM, DoD, inkjet) was not reported. The 3D-printed electrochemical biosensors for single and multi-sample analysis were developed to study the individual and combined toxicity of three different mycotoxin models, such as deoxynivalenol (DON) and its acetylated derivatives (3-ADON and 15-ADON), providing a potential advantage over traditional analysis. The sensing capability of the biosensor was validated on a linear range of 0.1–10 ppb, 0.05–100 ppb, and 0.1–10 ppb for DON, 3-ADON, and 15-ADON, respectively, with limits of detection equal to 0.07 ppb (DON), 0.10 ppb (3-ADON), and 0.06 ppb (15-ADON). Such analytical performance is abundantly suitable with regard to the limits and regulations of mycotoxins in food and feed to safeguard consumer health and avoid foodborne diseases [59].
2.4. Material Extrusion Applied to Environmental Safety Monitoring
The awareness concerning the urgent need to preserve environmental safety is clear, now more than ever. 3D printing technologies demonstrate their usefulness even in this case. In the following paragraph, different applications regarding environmental safety monitoring are reported. Considering the actual widespread use of herbicides in both agriculture and gardening, the gathered data about water pollution are putting the spotlight on the environmental damage and safety issues related to the overuse and the non-optimal management of these chemicals. In this scenario, developing affordable and reliable portable devices for the detection of these pollutants is becoming increasingly urgent. In response to this need, Ruan et al. [17] employed FDM 3D printing to design a PLA-based electrochemical immunosensing system for environmental safety monitoring. In particular, they implemented a portable biosensor for the simultaneous quantification of two classes of widespread herbicides in agricultural practices: atrazine and acetochlor. FDM was selected amongst other approaches thanks to its advantages in terms of cost–reliability ratio, compared with other techniques such as SLA and inkjet printing [48]. To enhance the biosensing ability of the system, antibodies was coupled with palladium–platinum nanoparticles (Pd@Pt NPs), relying on the relevant catalytic activity of the latter to improve the quantitative detection of herbicides. The final architecture was called NEMEIS, which represents the acronym of nanomaterial-enhanced multiplexed electrochemical immunosensing system. The device was composed of a strip cutter, a strip holder into which to plug the bifurcated immunochromatographic strip (ITS), and an electrode holder with two built-in reaction cells, with an overall size of 80 mm × 50 mm × 15 mm to obtain a system based on multiplex competitive lateral flow immunoassay (LFIA). Deepening the principle behind this device, the ITS was loaded with anti-atrazine and anti-acetochlor antibodies for the specific detection of these herbicides conjugated with mesoporous core-shell palladium@platinum nanoparticles (Ab-Pd@Pt NPs); the Pd@Pt NPs were employed for their significant peroxidase-like properties. This customized lateral-flow immunoassay enhanced the sensitivity of the analyzer, allowing the simultaneous determination of atrazine and acetochlor. The number of analytes bound on the Ab-Pd@Pt NPs was determined through electrochemical assay, measuring the general catalytic activity on the redox reaction between thionin acetate and hydrogen peroxide (H2O2). Pd@Pt NPs are capable of oxidizing thionine in the presence of H2O2, and the increased amount of the thionine oxidized form is measurable with cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The peak current signal is negatively correlated with the amount of herbicides contained in the sample. Following this correlation, it is possible to determine the concentration of analytes. The NEMEIS system was based on lateral-flow immunoassay; this technique was selected due to its affordability and portability and its simplicity of usage. The data elaboration allowed the setting of a limit of detection of 0.24 ppb and 3.2 ppb for atrazine and acetochlor, respectively, in a linear range of 0.1 to 500 ppb (atrazine) and 1 to 1000 ppb (acetochlor). The limit of detection shown by the device was comparable with the value achieved with a standard method, such as HPLC for atrazine determination (0.1 ppb) and GC/MS for acetochlor (10 ppb); in addition, the analytical performance was consistent with the EU limits of herbicides in drinking water (0.5 ppb for the total amount). The valuable results obtained by this electrochemical 3D-printed biosensor unravel the wide potential of 3D-printed biosensors for different purposes, such as portable monitoring and diagnostics [17].
Silva et al. [18] reported the development of an electrochemical-based sensor for the voltametric quantification of serotonin in synthetic urine and catechol detection in artesian water, employing reduced graphene embedded into polylactic acid (rGO-PLA) for the manufacture of three different electrodes (working, pseudo-reference, and counter electrodes) printed using fused deposition modelling (FDM). In this work, the use of conductive thermoplastic filaments for 3D printing applications was evaluated for a human health and environmental survey. The 3D-printed working electrode was subsequently subjected to two sequential treatments, using nitric acid and sodium borohydride to obtain rGO-PLA starting from graphene-PLA (G-PLA) 3D-printed electrodes. This procedure was reported to significantly improve the electrochemical properties of the final apparatus, and this experimental evidence was demonstrated by the differential-pulse-voltammetry measurement performed for serotonin detection and in tyrosinase-based voltametric analysis employed for catechol quantification in water samples. In both applications, the platform was demonstrated to be perfectly suitable for sensing and biosensing purposes, reporting a limit of detection equal to 0.032 µmol/L for serotonin quantification and 0.26 µmol/L for catechol. Particular mention should be given to the commitment that Silva et al. have shown in the development of a new procedure that avoids the use of toxic organic solvent, following the principles of green chemistry. João et al. [19] reported a manufacturing process using FDM for 3D-printed biodegradable electrodes made with carbon black/polylactic acid (CB-PLA) that could be used for quality control of bioethanol fuel. The working electrode was constructed by modelling the thermoplastic conductive filament (CB-PLA) by the extruder with a hot nozzle at 220 °C; the electrodes were 3D-printed with a hollow cube shape. The preparation of the CB-PLA 3D-printed electrode was made following this procedure: the four sides of the hollow cube were cut with scissors, giving rectangular pieces; subsequently, one side was manually polished with abrasive paper and then moistened with deionized water to control carbon black exposure; and the surface of the rectangular pieces was smoothened to avoid possible leaks when the electrode was coupled to the 3D-printed electrochemical cell. The coupling was realized by fixing the polished rectangular CB-PLA electrode over a stainless-steel plate to create a conductive surface; the electrode was then fixed using 3D-printed screw threads with a 3D-printed electrochemical cell to obtain the definitive architecture of the apparatus. The final step of fabrication involved the application of an electric potential to the electrode imbued in NaOH medium; this step was performed to remove the non-conductive polymeric material (PLA) from the working electrode surface, facilitating the electron transfer. The 3D-printed sensor was used for copper determination in bioethanol, as such metallic contaminants can be found in significant quantity in biofuels due to contamination that occurs during production, transportation, and storage. Copper ions act as a catalyst in the oxidation processes of fuel, leading to the formation of conglomerates that may obstruct motor components, such as engine pipes and injectors. The determination of copper was made using square-wave anodic-stripping voltammetry (SWASV). The 3D-printed CB-PLA electrode showed solid analytical performance in terms of limits of detection (LOD) and quantification (LOQ), with values equal to 0.097 µg/L and 0.323 µg/L, respectively, in a linear range of 10 to 300 µg/L. Optimal inter-day precision was reported (8%) and was calculated based on ten measurements performed on a copper concentration of 20 µg/L, in addition to recovery values between 95% and 103% obtained from the copper determination of spiked samples of biofuel. Bioethanol production and commercialization depends on strict quality control. In order to produce high-quality biofuel, the development of affordable and efficient devices capable of evaluating parameters such as metallic contaminant levels is essential for promoting the reduction in greenhouse gases (GHG) linked to the combustion of fossil fuels. Lupan et al. [20] reported the use of 3D inkjet DIW technology to manufacture nanocrystalline films for sensing electrolyte vapor from lithium-ion batteries (LIBs). 3D inkjet DIW was employed for the fabrication of Al2O3/CuO and CuO/Fe2O3 heterostructures, followed by an additional atomic layer deposition and thermal annealing step. Copper and iron nanoparticles were selected in this scenario, due to their ability to form oxide nanowires of CuO and nanoflakes of Fe2O3. These two oxide metals are, respectively, p-type and n-type semiconductors. This means that they can form p–n junctions, which have space charge regions that are capable of detecting changes in the electronic configuration of a single constituent, making them suitable for electrolyte vapor detection. Gas response was evaluated in response to different temperature conditions; the temperature-dependent detection was not equal for all the electrolytes. The sensing properties of these 3D-DIW-printed heterostructures proved their ability to detect, in relative concentrations, common electrolyte vapors released from LIBs. The 3D printing of nanostructures opened a new horizon in material science and nanoelectronics [20].
References
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980.
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat. Anal. Chem. 2021, 93, 3331–3336.
- Wang, L.; Gao, W.; Ng, S.; Pumera, M. Chiral Protein-Covalent Organic Framework 3D-Printed Structures as Chiral Biosensors. Anal. Chem. 2021, 93, 5277–5283.
- Cheng, H.; Yi, L.; Wu, J.; Li, G.; Zhao, G.; Xiao, Z.; Hu, B.; Zhao, L.; Tian, J. Drug Preconcentration and Direct Quantification in Biofluids Using 3D-Printed Paper Cartridge. Biosens. Bioelectron. 2021, 189, 113266.
- Pantazis, A.K.; Papadakis, G.; Parasyris, K.; Stavrinidis, A.; Gizeli, E. 3D-Printed Bioreactors for DNA Amplification: Application to Companion Diagnostics. Sens. Actuators B Chem. 2020, 319, 128161.
- Mojena-Medina, D.; Hubl, M.; Bäuscher, M.; Jorcano, J.L.; Ngo, H.-D.; Acedo, P. Real-Time Impedance Monitoring of Epithelial Cultures with Inkjet-Printed Interdigitated-Electrode Sensors. Sensors 2020, 20, 5711.
- Parate, K.; Rangnekar, S.V.; Jing, D.; Mendivelso-Perez, D.L.; Ding, S.; Secor, E.B.; Smith, E.A.; Hostetter, J.M.; Hersam, M.C.; Claussen, J.C. Aerosol-Jet-Printed Graphene Immunosensor for Label-Free Cytokine Monitoring in Serum. ACS Appl. Mater. Interfaces 2020, 12, 8592–8603.
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647.
- Contreras-Naranjo, J.E.; Perez-Gonzalez, V.H.; Mata-Gómez, M.A.; Aguilar, O. 3D-Printed Hybrid-Carbon-Based Electrodes for Electroanalytical Sensing Applications. Electrochem. Commun. 2021, 130, 107098.
- Crevillen, A.G.; Mayorga-Martinez, C.C.; Zelenka, J.; Rimpelová, S.; Ruml, T.; Pumera, M. 3D-Printed Transmembrane Glycoprotein Cancer Biomarker Aptasensor. Appl. Mater. Today 2021, 24, 101153.
- He, Z.; Huffman, J.; Curtin, K.; Garner, K.L.; Bowdridge, E.C.; Li, X.; Nurkiewicz, T.R.; Li, P. Composable Microfluidic Plates (CPlate): A Simple and Scalable Fluid Manipulation System for Multiplexed Enzyme-Linked Immunosorbent Assay (ELISA). Anal. Chem. 2021, 93, 1489–1497.
- Samarentsis, A.G.; Pantazis, A.K.; Tsortos, A.; Friedt, J.-M.; Gizeli, E. Hybrid Sensor Device for Simultaneous Surface Plasmon Resonance and Surface Acoustic Wave Measurements. Sensors 2020, 20, 6177.
- Sibug-Torres, S.M.; Go, L.P.; Enriquez, E.P. Fabrication of a 3D-Printed Porous Junction for Ag|AgCl|gel-KCl Reference Electrode. Chemosensors 2020, 8, 130.
- Bai, Y.; Zhang, D.; Guo, Q.; Xiao, J.; Zheng, M.; Yang, J. Study of the Enzyme Activity Change Due to Inkjet Printing for Biosensor Fabrication. ACS Biomater. Sci. Eng. 2021, 7, 787–793.
- Niaraki Asli, A.E.; Guo, J.; Lai, P.L.; Montazami, R.; Hashemi, N.N. High-Yield Production of Aqueous Graphene for Electrohydrodynamic Drop-on-Demand Printing of Biocompatible Conductive Patterns. Biosensors 2020, 10, 6.
- Wei, K.; Sun, J.; Gao, Q.; Yang, X.; Ye, Y.; Ji, J.; Sun, X. 3D “Honeycomb” Cell/Carbon Nanofiber/Gelatin Methacryloyl (GelMA) Modified Screen-Printed Electrode for Electrochemical Assessment of the Combined Toxicity of Deoxynivalenol Family Mycotoxins. Bioelectrochemistry 2021, 139, 107743.
- Ruan, X.; Wang, Y.; Kwon, E.Y.; Wang, L.; Cheng, N.; Niu, X.; Ding, S.; Van Wie, B.J.; Lin, Y.; Du, D. Nanomaterial-Enhanced 3D-Printed Sensor Platform for Simultaneous Detection of Atrazine and Acetochlor. Biosens. Bioelectron. 2021, 184, 113238.
- Silva, V.A.O.P.; Fernandes-Junior, W.S.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-Printed Reduced Graphene Oxide/Polylactic Acid Electrodes: A New Prototyped Platform for Sensing and Biosensing Applications. Biosens. Bioelectron. 2020, 170, 112684.
- João, A.F.; Squissato, A.L.; Richter, E.M.; Muñoz, R.A.A. Additive-Manufactured Sensors for Biofuel Analysis: Copper Determination in Bioethanol Using a 3D-Printed Carbon Black/Polylactic Electrode. Anal. Bioanal. Chem. 2020, 412, 2755–2762.
- Lupan, O.; Krüger, H.; Siebert, L.; Ababii, N.; Kohlmann, N.; Buzdugan, A.; Bodduluri, M.T.; Magariu, N.; Terasa, M.-I.; Strunskus, T.; et al. Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring. Chemosensors 2021, 9, 252.
- Lehman, S.E.; McCracken, J.M.; Miller, L.A.; Jayalath, S.; Nuzzo, R.G. Biocompliant Composite Au/PHEMA Plasmonic Scaffolds for 3D Cell Culture and Noninvasive Sensing of Cellular Metabolites. Adv. Healthc. Mater. 2021, 10, 2001040.
- Li, X.; Pan, X.; Lu, J.; Zhou, Y.; Gong, J. Dual-Modal Visual/Photoelectrochemical All-in-One Bioassay for Rapid Detection of AFP Using 3D Printed Microreactor Device. Biosens. Bioelectron. 2020, 158, 112158.
- Cao, L.; Han, G.-C.; Xiao, H.; Chen, Z.; Fang, C. A Novel 3D Paper-Based Microfluidic Electrochemical Glucose Biosensor Based on RGO-TEPA/PB Sensitive Film. Anal. Chim. Acta 2020, 1096, 34–43.
- Mao, D.; Li, W.; Zhang, F.; Yang, S.; Isak, A.N.; Song, Y.; Guo, Y.; Cao, S.; Zhang, R.; Feng, C.; et al. Nanocomposite of Peroxidase-Like CucurbitUril with Enzyme-Encapsulated ZIF-8 and Application for Colorimetric Biosensing. ACS Appl. Mater. Interfaces 2021, 13, 39719–39729.
- Dubbin, K.; Dong, Z.; Park, D.M.; Alvarado, J.; Su, J.; Wasson, E.; Robertson, C.; Jackson, J.; Bose, A.; Moya, M.L.; et al. Projection Microstereolithographic Microbial Bioprinting for Engineered Biofilms. Nano Lett. 2021, 21, 1352–1359.
- Sharafeldin, M.; Chen, T.; Ozkaya, G.U.; Choudhary, D.; Molinolo, A.A.; Gutkind, J.S.; Rusling, J.F. Detecting Cancer Metastasis and Accompanying Protein Biomarkers at Single Cell Levels Using a 3D-Printed Microfluidic Immunoarray. Biosens. Bioelectron. 2021, 171, 112681.
- Hart, C.; Didier, C.M.; Sommerhage, F.; Rajaraman, S. Biocompatibility of Blank, Post-Processed and Coated 3D Printed Resin Structures with Electrogenic Cells. Biosensors 2020, 10, 152.
- Park, C.; Abafogi, A.T.; Ponnuvelu, D.V.; Song, I.; Ko, K.; Park, S. Enhanced Luminescent Detection of Circulating Tumor Cells by a 3D Printed Immunomagnetic Concentrator. Biosensors 2021, 11, 278.
- Sharafeldin, M.; James, T.; Davis, J.J. Open Circuit Potential as a Tool for the Assessment of Binding Kinetics and Reagentless Protein Quantitation. Anal. Chem. 2021, 93, 14748–14754.
- Zheng, L.; Cai, G.; Qi, W.; Wang, S.; Wang, M.; Lin, J. Optical Biosensor for Rapid Detection of Salmonella Typhimurium Based on Porous Nanocatalysts and a 3D Fluidic Chip. ACS Sens. 2020, 5, 65–72.
- Calabria, D.; Guardigli, M.; Severi, P.; Trozzi, I.; Pace, A.; Cinti, S.; Zangheri, M.; Mirasoli, M. A Smartphone-Based Chemosensor to Evaluate Antioxidants in Agri-Food Matrices by In Situ AuNP Formation. Sensors 2021, 21, 5432.
- Adamopoulos, C.; Gharia, A.; Niknejad, A.; Stojanović, V.; Anwar, M. Microfluidic Packaging Integration with Electronic-Photonic Biosensors Using 3D Printed Transfer Molding. Biosensors 2020, 10, 177.
- Jordan, R.S.; Frye, J.; Hernandez, V.; Prado, I.; Giglio, A.; Abbasizadeh, N.; Flores-Martinez, M.; Shirzad, K.; Xu, B.; Hill, I.M.; et al. 3D Printed Architected Conducting Polymer Hydrogels. J. Mater. Chem. B 2021, 9, 7258–7270.
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D Printed Microfluidic Devices for Circulating Tumor Cells (CTCs) Isolation. Biosens. Bioelectron. 2020, 150, 111900.
- Terutsuki, D.; Mitsuno, H.; Kanzaki, R. 3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging. Sensors 2020, 20, 5779.
- Arshavsky-Graham, S.; Enders, A.; Ackerman, S.; Bahnemann, J.; Segal, E. 3D-Printed Microfluidics Integrated with Optical Nanostructured Porous Aptasensors for Protein Detection. Microchim. Acta 2021, 188, 67.
- Siller, I.G.; Preuss, J.-A.; Urmann, K.; Hoffmann, M.R.; Scheper, T.; Bahnemann, J. 3D-Printed Flow Cells for Aptamer-Based Impedimetric Detection of E. Coli Crooks Strain. Sensors 2020, 20, 4421.
- Agostino, V.; Massaglia, G.; Gerosa, M.; Sacco, A.; Saracco, G.; Margaria, V.; Quaglio, M. Environmental Electroactive Consortia as Reusable Biosensing Element for Freshwater Toxicity Monitoring. New Biotechnol. 2020, 55, 36–45.
- Achille, C.; Parra-Cabrera, C.; Dochy, R.; Ordutowski, H.; Piovesan, A.; Piron, P.; Van Looy, L.; Kushwaha, S.; Reynaerts, D.; Verboven, P.; et al. 3D Printing of Monolithic Capillarity-Driven Microfluidic Devices for Diagnostics. Adv. Mater. 2021, 33, 2008712.
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.; Kwon, S.J.; Cho, J.H. 3D-Printed Sugar Scaffold for High-Precision and Highly Sensitive Active and Passive Wearable Sensors. Adv. Sci. 2020, 7, 1902521.
- Kanitthamniyom, P.; Zhou, A.; Feng, S.; Liu, A.; Vasoo, S.; Zhang, Y. A 3D-Printed Modular Magnetic Digital Microfluidic Architecture for on-Demand Bioanalysis. Microsyst. Nanoeng. 2020, 6, 48.
- Aggas, J.R.; Abasi, S.; Phipps, J.F.; Podstawczyk, D.A.; Guiseppi-Elie, A. Microfabricated and 3-D Printed Electroconductive Hydrogels of PEDOT:PSS and Their Application in Bioelectronics. Biosens. Bioelectron. 2020, 168, 112568.
- Khosravani, M.R.; Reinicke, T. 3D-Printed Sensors: Current Progress and Future Challenges. Sens. Actuators A Phys. 2020, 305, 111916.
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1701161.
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D Printed Medicines: A New Branch of Digital Healthcare. Int. J. Pharm. 2018, 548, 586–596.
- Ferretti, P.; Santi, G.M.; Leon-Cardenas, C.; Freddi, M.; Donnici, G.; Frizziero, L.; Liverani, A. Molds with Advanced Materials for Carbon Fiber Manufacturing with 3D Printing Technology. Polymers 2021, 13, 3700.
- Napadensky, E. Inkjet 3D Printing. In The Chemistry of Inkjet Inks; World Scientific: Singapore, 2009; p. 255. ISBN 978-981-281-821-828.
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet Printing of Proteins. Soft Matter 2009, 5, 4866.
- Secor, E.B. Principles of Aerosol Jet Printing. Flex. Print. Electron. 2018, 3, 035002.
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 1–10.
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A Review of 3D Printing Techniques for Environmental Applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178.
- Liu, Z.; Zhang, Y.; Xu, S.; Zhang, H.; Tan, Y.; Ma, C.; Song, R.; Jiang, L.; Yi, C. A 3D Printed Smartphone Optosensing Platform for Point-of-Need Food Safety Inspection. Anal. Chim. Acta 2017, 966, 81–89.
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153.
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3D Printed Chitosan-Based Scaffolds Containing α-Tocopherol Showing Antioxidant and Antimicrobial Activity. Appl. Sci. 2021, 11, 7253.
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844.
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D Printed Chitosan Scaffolds: A New TiO2 Support for the Photocatalytic Degradation of Amoxicillin in Water. Water Res. 2019, 163, 114841.
- Zanotti, I.; Marando, S.; Remaggi, G.; Bergonzi, C.; Bernini, F.; Bettini, R.; Elviri, L. The Adaptation of Lipid Profile of Human Fibroblasts to Alginate 2D Films and 3D Printed Scaffolds. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129734.
- Guo; Niaraki Asli; Williams; Lai; Wang; Montazami; Hashemi Viability of Neural Cells on 3D Printed Graphene Bioelectronics. Biosensors 2019, 9, 112.
- Mazumder, P.M.; Sasmal, D. Mycotoxins—Limits and Regulations. Anc. Sci. Life 2001, 20, 1–19.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
18 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




