Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shadma Wahab | + 4253 word(s) | 4253 | 2022-01-04 10:54:41 | | | |
| 2 | Vivi Li | + 103 word(s) | 4356 | 2022-02-18 03:00:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wahab, S. Pharmacological Efficacy of Tamarix aphylla. Encyclopedia. Available online: https://encyclopedia.pub/entry/19586 (accessed on 08 February 2026).
Wahab S. Pharmacological Efficacy of Tamarix aphylla. Encyclopedia. Available at: https://encyclopedia.pub/entry/19586. Accessed February 08, 2026.
Wahab, Shadma. "Pharmacological Efficacy of Tamarix aphylla" Encyclopedia, https://encyclopedia.pub/entry/19586 (accessed February 08, 2026).
Wahab, S. (2022, February 17). Pharmacological Efficacy of Tamarix aphylla. In Encyclopedia. https://encyclopedia.pub/entry/19586
Wahab, Shadma. "Pharmacological Efficacy of Tamarix aphylla." Encyclopedia. Web. 17 February, 2022.
Copy Citation
Tamarix aphylla is a well-known species of the genus Tamarix. T. aphylla (Tamaricaceae) is a perennial tree in Asia, the Middle East, and Central Africa. It is used as a carminative diuretic in tuberculosis, leprosy, and hepatitis. Various pharmacological properties have been shown by T. aphylla, such as antidiabetic, anti-inflammatory, antibacterial, antifungal, anticholinesterase, and wound-healing activity. However, T. aphylla has not received much attention for its secondary metabolites and bioactive constituents. Research has shown that this plant has hidden potential that needs to be explored.
T. aphylla
phytochemicals
biological activity
anti-inflammatory
antidiabetic
antibacterial
antifungal
anticholinesterase
1. Introduction
Natural product-based medication has been a source of treatment for various ailments for thousands of years [1]. Herbal medicines have long played a vital role in developing drugs to treat a variety of diseases. Currently, available medicines are distinctions or reproductions of constituents, combinations, and dilutions that exist in nature [2]. People’s interest in herbal medicines is currently increasing due to fewer or no side-effects [3]. Despite the minimal risk of adverse effects, the possibility of a medication interaction cannot be rejected. Another appealing attribute for accepting a comprehensive collection of herbal medications is cost-effectiveness. The use of herbal medicines is historical, but there is a space in ancient traditional practices fulfilled by alternative medicine and complementary medicines incorporating new technologies [4][5][6]. Therefore, researchers should continue to research new plant-based therapy from the vast reservoir of nature [7][8].
Tamaricaceae (Caryophyllales) is a family of around 80 rheophytes, halophytes, and xerophytes grown in semi-arid and dry regions, especially in central and southwest Asia [9][10]. It is a native plant of African and Asian countries used by the locals for medicinal purposes and is commonly called tamarisk [11][12]. This plant has needle-like leaves coated in salt secreted by the salt glands [13]. The leaf attachment on the stem and the shape of the androecium are the most significant distinguishing features of Tamarix species: shrubs and trees. Some morphological characteristics have intermediate states of Tamarix; therefore, it is the most taxonomically challenging genus among angiosperms [9][10]. The reported phytochemical work on Tamarix species has shown that the main chemical constituents are polyphenolic constituents such as flavonoids, tannins, and phenolic acids [12]. The phytochemical research revealed that all extracts were noticeably devoid of alkaloids, followed by the presence of tannins. In addition, different parts of the plant have reported flavonoids, steroids, cardiac glycosides, and derivatives of ellagic acid and gallic acid [14][15][16][17][18][19]. T. aphylla has long been used as an antipyretic, analgesic, antirheumatic, and anti-inflammatory. Gall extract has been used for throat infections and temporarily constricting the vaginal mucous membrane before intercourse [4][14][20][21][22]. The bark of this plant is used to cure skin-related diseases, and gall on the flowers is used for its astringent effect [23]. There are various folk uses such as carminative, eczema, antimicrobial, antioxidant, aphrodisiac, anthelmintic, diuretic, anti-hemorrhoid, antidiarrheal, and skin diseases. In addition, many other studies have exhibited activities toward inflammation, joint pain, and internal tumors [20][24]. A complete overview of the T. aphylla’s ethnobotanical, phytochemical, and pharmacological potential is shown in Figure 1.

Figure 1. Overview of the T. aphylla ethnobotanical, phytochemical, and pharmacological potential.
2. Ethnopharmacology: Traditional Practices
T. aphylla (Tamaricaceae) is the most well-known Tamarix species, reaching up to 18 m (60 feet) high. This plant is known with different names such as saltcedar, Athel tree, Athel tamarisk, and Athel pine. It is a treasure of various regions of the world including Central Africa, the Middle East, and Asia. Species of Tamarix grow in dry and hot climates, while some of its species are also found in moderate temperatures [12][13][25]. Ethnopharmacological reports on T. aphylla are presented in Table 1.
Table 1. T. aphylla’s ethnomedicinal usage in many places around the world.
| Geographical Location | Parts of the Plant Used | Indication | Route of Administration | Results | References |
|---|---|---|---|---|---|
| Southeastern Morocco | Leaf | Hypertension | S/Decoction | Information was collected from the respondents of Errachidia province regarding the plants used for hypertension, one of which was T. aphylla. | [26] |
| The central region of Abyan governorate, Yemen | Bark and leaf | Abdominal pain, hair loss, cough, and asthma | S, Lo/Infusion, decoction | Ethnobotanical survey of medicinal plants showed that residents of Yemen use T. aphylla for abdominal pain, hair loss, cough, and asthma. | [27] |
| Northwestern part of Pakistan | Whole plant | Abscesses and wounds, rheumatism, jaundice, bad evils | S, Lo/Decoction of ash, ash, boiled leaves | T. aphylla is one of the plants enlisted in the Holy Quran, Islamic literature, and Ahadith with ethnobotanical relevance. | [20] |
| District Sargodha, Punjab, Pakistan | Bark | Measles, aphrodisiac | S/Powdered with oil, smoke | T. aphylla is one of the most used timber species in district Sargodha. | [28] |
| Peshawar Valley of Pakistan | Bark and leaf | Paralysis, abdominal pain, tetanus, rheumatism, and wound healing | S, Lo/Powder extract | Ethnobotanical study on medicinal plants exhibited that residents of Peshawar use T. aphylla in various diseases. | [29] |
| District Karak, Pakistan | Leaf | Animal pain killer for wounds, in bird flu | S/Smoke | This study showed the ethnoveterinary use of T. aphylla. | [30] |
| Pakistan | Fruit | Diabetes | S/Decoction | T. aphylla can be used as antidiabetic medication. | [31] |
| Karamar Valley, Swabi, Pakistan | Bark | Jaundice, rheumatism, infection of gums and teeth | ND | T. aphylla’s extract showed activity against the biofilm-causing bacteria in periodontal diseases. | [32] |
| Chenab riverine area, Punjab province, Pakistan | Leaf and bark | Cough and cold, eye infection, wounds and boils, febricity |
S, Lo/ Poultice, paste, decoction, ash |
Local people employ T. aphylla to cure various ailments in numerous regions of Pakistan. | [33] |
| Rajhan Pur, Punjab, Pakistan |
Root, leaf | All contagious diseases, jaundice, smallpox, leprosy, tuberculosis | ND | A field survey showed various uses of T. aphylla in multiple diseases. | [34] |
| Central Sahara | Shoots | Aid to menstruation, postpartum care, fever |
S/Decoction | Results showed that people traditionally use T. aphylla as a potential traditional healer. | [35] |
| Jordan, North Badia | Leaf | Fever | S/Decoction | T. aphylla is an effective medication in pain and inflammations. | [36] |
Abbreviations: T: Tamarix, L: leaf, B: bark, F: fruit, G: gall, R: root, SH: shoots, WP: whole plant, S: systemic, Lo: local, ND: not defined.
Most ethnopharmacological studies were published in Pakistan. These studies have shown that T. aphylla is used to treat various infectious diseases, including dental infections, smallpox, leprosy, tuberculosis, colds, and coughs [33][32][34]. Many other communications from other parts of the world have established that T. aphylla has antidiabetic, antibacterial, anti-inflammatory, and antifungal properties, in addition to being effective in periodontal disease, along with anticholinesterase and wound-healing activities. These activities are due to the tremendous number of phenolic compounds with astringent effects. Tamarix aphylla, together with the Aerva javanica plant, was studied ethnobotanically, chemically, and biologically in the Asir region of Saudi Arabia. The study claimed that both plants had a high usage value (UV), indicating knowledge of the medical relevance and uses of these plants. In the plains and valleys (wadis) of the Asir region, T. aphylla is ubiquitous, natural, and well-grown. T. aphylla’s different parts were reported to be used in various ailments, including joint pain, skin issues, and kidney problems. Plants with a high UV reflect their extensive use by locals in the area with several therapeutic effects toward human health issues. As a result, the author reported that bioactive phytochemicals and other pharmacological actions should be investigated in these plants [37].
Islamic religious scripture, Holy Quran, recognizes T. aphylla with names such as Athel or Tarfaa. As a useful traditional phytotherapy for jaundice, T. aphylla leaf ash is mixed with water, which is filtered and boiled after half an hour. Salt is left behind after water evaporation, of which 0.5–1 g is consumed with Shurbat-e-Bazoori twice daily [23]. Traditionally, the plant is known as “Mayyin Khurd” in Unani, Macheeka in Ayurveda, and Sivappattushavukku in Siddha, among other names. According to the history of folk medicinal usage, the plant has been used for antirheumatic, analgesic, and antipyretic purposes [4]. The folkloric assertion shows the use of T. aphylla in fever, inflammation, and pain, in addition to antimicrobial, antidiarrheal, anti-hemorrhoid, and antioxidant activities, along with use in eczema and skin diseases, as well as an aphrodisiac, diuretic, carminative, and anthelmintic [21][23][24].To cure wounds, abscesses, and rheumatism, the leaves are boiled in water, and water is strained; then, hot leaves are tied with the affected area. This therapy is continued for a week. The galls are astringent, and their aqueous extract is used as a gargle to cure throat infections. An extract of the leaves is used to treat toothache [14][38]. Ethnobotanical uses include the treatment of ulcer, GIT disorders, epilepsy, hair loss, and various dermatological problems [39][40]. There is a definitive history of using root decoctions to cure contagious ailments, such as smallpox, tuberculosis, and leprosy. The tree’s young branch and leaf decoction are used for tetanus, spleen, and gynecological problems [41].
Tamarix aphylla grows widely in diverse parts of the world; therefore, its vernacular names differ: German, Tamariske; Spanish, Taray; French, Tamaris; Arabic, Abal, Tarfaa, Ghaz, and Athel; Afrikaans, Woestyntamarisk; English, Athel pine, saltcedar, Tamarix, tamarisk, desert Tamarix, Athel tamarisk, and Athel tree [42][43][44].
3. Phytochemistry
This section of the entry describes the structures of many secondary metabolites of T. aphylla and the functions of these metabolites in plants and humans. These compounds can be divided into four major biosynthetic classes: phenylpropanoids, terpenoids, alkaloids, and polyketides. The study of phytochemicals has led to drug discovery. The available literature on T. aphylla focuses on the characterization and isolation of particular parts of this plant [14][18][45][46]. A combination of phenolic chemicals characterizes this plant material [47]. There are several uses of T. aphylla, and its preparations are used as natural remedies to treat many diseases. Unfortunately, most of the studies have not thoroughly investigated the phytochemicals of T. aphylla. Most research has been conducted on the structural characterization and isolation of chemical components, including gallic acid, ellagic acid, and flavonoids [14][18][45][47][48][49]. Tannins and flavonoid-rich sources have been found in T. aphylla leaves [50].
Flavonoids and alkaloids extracted from T. aphylla have been examined for antibacterial activity. Phytochemical screening of most extracts of T. aphylla has exhibited the presence of tannins and a lack of alkaloids. Bioactive mixtures and metabolites have been found, such as cardiac glycosides, flavonoids, terpenoids, and steroids. Gallic and ellagic acid are derivatives of this plant species [15][16][17][19]. The leaves and stems of T. aphylla have a comparable phytochemical composition. Nevertheless, quantitative analyses have indicated that the leaves have a considerably greater quantity of polyphenols than the stems [17]. A floral extract of T. aphylla yielded isoferulic acid, 3-O-beta-glucopyranoside, glycosylated isoferulic acid, and novel phenolics such as dehydrodigallic acid dimethyl ester and tamarixetin 3,3’-disodium sulfate. According to the spectral data, their structures were developed [18]. Various studies have shown structural derivatives among hydrolyzable tannins [51]. T. aphylla galls, on the other hand, generate the ellagitannin tamarixellagic acid [49]. According to a study, under an abiotic stress environment, a halophytic plant stimulates oxidative stress reactive oxygen species in hostile conditions. Therefore, time and collection area are factors that may significantly change the amounts of secondary metabolites [17][49][52].
4. Pharmacological Efficacy of Tamarix aphylla
4.1. Anti-Inflammatory Activity
Inflammation is the body’s reaction to fight burns, injuries, toxic stimuli, and infections that emerge as anorexia, fever, and edema. Inflammation is the body’s first defense reaction that removes undesirable effects and commences the healing operations. This protective system is crucial for a healthy and normal physiological state. Inflammation can be acute or chronic [53][54]. Removing the damaged stimuli is the treatment of acute inflammatory conditions; however, unchecked acute inflammation can lead to chronic inflammation. A chronic inflammatory response can cause serious health problems, such as neurological and cardiovascular diseases and cancer [55]. Alzheimer’s disease, cardiovascular disease, metabolic disorders, allergies, malignancies, and autoimmune illnesses all have chronic inflammation as a contributing factor [56]. Anti-inflammatory medications, both nonsteroidal and steroidal, are the most prescribed treatments. In most documented cases, the long-term use of these medicines is hampered by their side-effects, necessitating the therapeutic use of less toxic solutions with fewer unpleasant responses [57][58]. Consequently, there is a requirement for effective, safe, and cost-effective alternative therapies.
Herbal therapy has been used to treat various ailments since ancient times, including chronic inflammatory diseases. Medicinal plants contain anti-inflammatory properties and have limited or no adverse effects [3]. T. aphylla is generally safe and harmless to humans. However, inflammation is a significant development in many illnesses [59]. Consequently, there is a persistent need to learn more about the benefits of natural medications in treating inflammatory diseases to expand researchers' scientific awareness. Tamarix species possess various compounds such as phenolic acids, tannins, sulfur-containing compounds, and flavonoids, which have been used since ancient times to cure chronic inflammatory disorders [11][12]. T. aphylla is an encouraging herbal medication with a wide range of metabolites, many medical uses, and pharmacological assurance. The aqueous ethanolic extract of T. aphylla galls has been shown to have anti-inflammatory and antipyretic properties [4]. The inflammatory pathway and probable role of T. aphylla as an anti-inflammatory drug are illustrated in Figure 2.
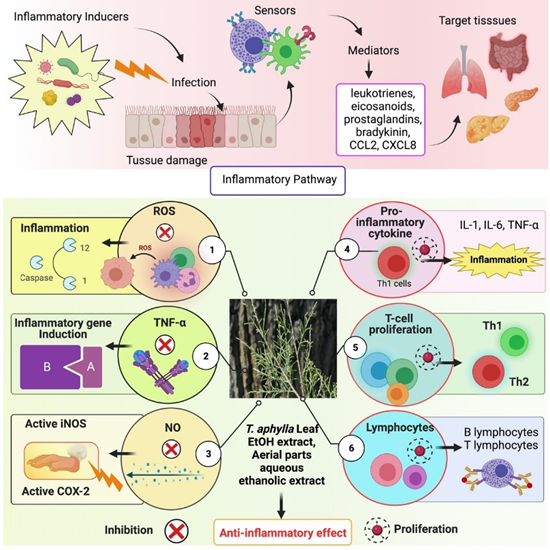
Figure 2. Inflammatory pathway and the probable role of T. aphylla as an anti-inflammatory agent. ROS: Reactive oxygen species, TNF-α: Tumor necrosis factor alpha, NO: Nitric oxide, IL-1: Interleukin-1, IL-6: Interleukin-6.
An investigation was carried out to examine the modulatory effect of T. aphylla, in which bioassay-guided isolation was carried out on several inflammatory indicators such as TNF-α and ROS, proliferation of lymphocyte T cells, and proinflammatory cytokines. The aqueous extract of T. aphylla suppressed intracellular ROS production, NO generation, and T-cell proliferation. The most effective inflammatory markers were the liquid–liquid fractional method partitioned aqueous ethanolic crude extract and n-BuOH and DCM extracts. In addition, 3,5-dihydroxy-4-methoxy benzoic acid methyl ester from the DCM extracts suppressed the generation of ROS and TNF-α. Furthermore, all mediators examined demonstrated inhibitory action in the presence of kaempferol. Therefore, T. aphylla constituents might be used as anti-inflammatory medications [58].
4.2. Wound-Healing Activity
The sequence of interconnected processes that replace damaged tissue at the injury site with newly produced tissue is called wound healing. Regular biological action is attained through four remarkably programmed stages: hemostasis, inflammation, proliferation, and remodeling. All four stages must happen in a certain time frame with a proper sequence to heal the wound. Many factors affect the process of wound healing, such as infection, oxygenation, sex hormones, age, stress, obesity, alcoholism, nutrition, smoking, and diabetes [60]. Various studies have proven that natural products affect all these factors. Natural therapeutics promote wound healing and fix impaired wounds with lessened side effects. Natural products have been used since ancient times to heal wounds due to their pharmacological importance, such as antibacterial, anti-inflammatory, and cell-stimulating potential [61]. The effectiveness of these natural products has been examined through in vitro, in vivo, and human studies. Skin wound healing is a perplexing biological activity and a coordinated cellular and biochemical sequence. Combining herbal materials with advanced drugs has improved regeneration [62].
T. aphylla’s anti-inflammatory and wound-healing properties are reported in Islamic literature and other resources from far-flung regions of Saudi Arabia. In the Al-Qassim region of Saudi Arabia, dried powders of all plant parts are used for 1 week to treat camel skin disorder (allergic or mycotic dermatitis) [63]. Burnt smoke from the dried pulverized plant leaves has history as an injury-healing agent and a dental painkiller [38]. It was found that boiling plant leaves and tying them to afflicted skin manages abscesses and rheumatism, in addition to wound healing [23]. An animal study examined an ethanolic extract of T. aphylla for wound-healing activity. A linear incision and a circular excision were employed to assess wound healing. Total protein estimation, antioxidant activity, lipid peroxides, uronic acid, hexosamine, and the tensile strength of tissue from incision wounds were examined. The period of epithelialization and TNF-α concentration of the wound tissues were also assessed in this study. Compared to conventional medication, the extract treatment groups exhibited considerable antibacterial activity. Results of the study showed that T. aphylla extract substantially enhanced the collagen content and remarkably increased tensile strength. T. aphylla extract has been shown to prevent oxidative damage, speed up healing activity, and have potent antioxidant properties. The epithelialization duration of the treated wounds was reduced by 40%. The study’s findings showed that the ethanolic extract of T. aphylla has strong wound-healing ability [64]. Another study investigated the wound-healing potential of T. aphylla extract by adopting the induced paw edema model in Wistar rats. In addition, the gel base of a T. aphylla extract, namely, Carbapol-934, was employed to investigate carrageen-induced anti-inflammatory properties. This examination showed that T. aphylla has wound-healing, anti-inflammatory, and antioxidant properties [65]. There is an urgent need to determine the actual mechanism of action, the safety, and the adverse effects of T. aphylla and its secondary metabolites; furthermore, there is a demand for revamped purification techniques, innovative extraction methods, and quality control evaluation with treatment regimes. Although T. aphylla is less expensive than current therapies, it is susceptible to geographic location, season, and batch-to-batch fluctuation, resulting in unanticipated allergic responses and adverse effects. The possible mechanism of T. aphylla wound-healing activity is shown in Figure 3.
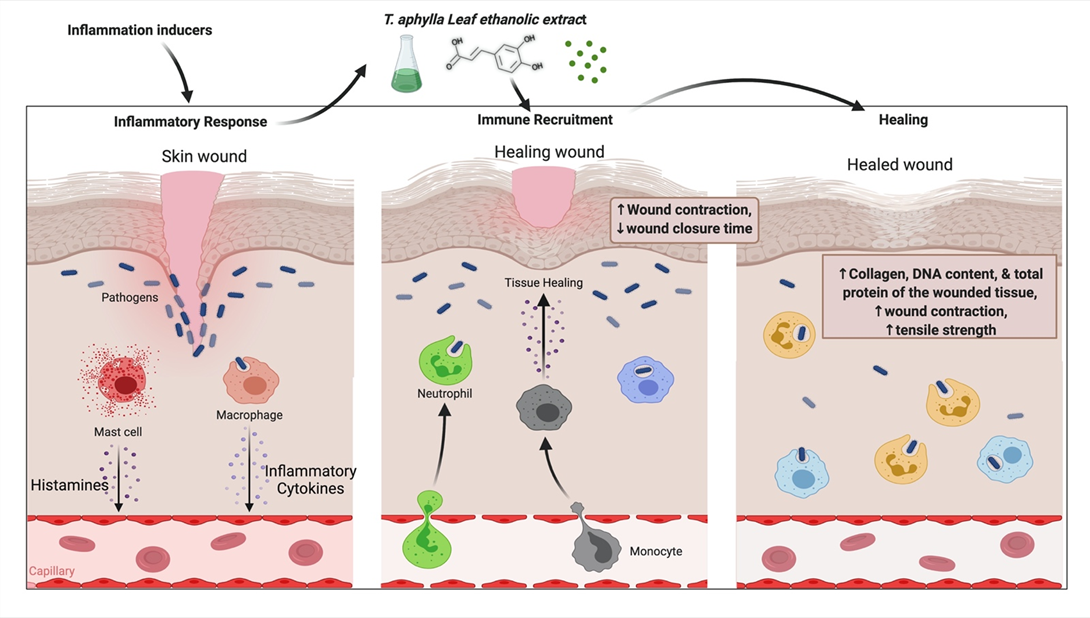
Figure 3. Probable mechanism of T. aphylla wound-healing activity.
4.3. Antibacterial Activity
In tropical and subtropical areas, human diseases, particularly those involving microorganisms such as bacteria, fungus, viruses, and nematodes, inflict significant harm. Over the last two decades, there has been a surge in interest in natural products as potential sources of novel antimicrobial agents. Consequently, various extracts from conventional herbal drugs have been examined to discover numerous pharmacological benefits [66][67][68]. Moreover, there is a need for novel herbal extracts and natural medicines that might effectively resist bacteria [66][69]. The antibacterial properties of plant phytochemicals have demonstrated their therapeutic usefulness in treating bacterial infections [70][71]. Therefore, documentation of the constituents and antibacterial activity of medicinal plants is precious since it may be utilized to conduct additional research to develop innovative antibacterial treatments.
Therefore, it is necessary to explore the phytochemical constituents of T. aphylla to treat infections [72]. This section aims to explore the antibacterial activities of extracts and secondary metabolites of T. aphylla to bridge existing research gaps, to provide suggestions based on the current understanding of T. aphylla’s advantages, and to advise more scientific research for innovative antibacterial treatments. All the secondary metabolites of T. aphylla listed in this entry have medicinal properties, thus authenticating their usage [73]. For future study and the formation of new antibiotics to fight resistant pathogenic bacteria, all related studies of T. aphylla are discussed.
The antibacterial activity of a methanolic extract of T. aphylla bark against microbial strains in a study provided scientific evidence for the use of its phytoconstituents as an antimicrobial treatment. Halophytic species with stimulant and hepatotoxic properties are used to treat liver-related ailments. Halophytic species suppressed all bacterial strains, leaving Listeria monocytogenes, and studies have shown that polar methanolic fractions are more effective than polar chloroform fractions [74]. A study was conducted to test the antibacterial activity against 10 pathogenic bacteria implicated in severe ailments affecting humans and animals. Fresh and disease-free T. aphylla leaves were accumulated from Saudi Arabia’s various geographical locations. The antibacterial screening effectiveness of T. aphylla leaves against multidrug-resistant human infections was studied in vitro. The antibacterial effects of the methanol and ethanol leaf extracts were different and showed varying inhibitory effects against the examined pathogenic strains, ranging from modest inhibition (4 ± 0.6 mm) to high inhibition (20.7 ± 1.3 mm). There were no significant variations in the lowest inhibitory concentrations of ethanol and methanol extracts. T. aphylla contains antibacterial biomolecules that might be used to combat multidrug-resistant human infections [75]. The methanolic extract of T. aphylla and its phytochemical constituents were explored in vitro for antibacterial activity against various microbial strains. The disc diffusion assay with 25, 50, 75, and 100 mg/mL stock solutions was employed to examine the antibacterial activity toward Escherichia coli, Bacillus subtilis, Salmonella typhi, and Staphylococcus aureus, in addition to antifungal activity toward strains such as Aspergillus flavus and Candida albicans. The extract’s inhibitory efficacy in millimeters was calculated by measuring the zone of growth inhibition surrounding the discs and comparing it to the reference medication. The study showed that T. aphylla bark extract has a more significant inhibition zone at higher concentrations [76]. These compelling results can motivate health workers to use bioactive materials and phytoconstituents.
Biofilms are multispecies bacterial colonies that invade the oral cavity as plaque, causing dental cavities and periodontal disease [77]. In one study, three herbs were examined to determine their effectiveness against pinpointed dental biofilm-forming bacteria. Pathogenic strains that form dental biofilms were isolated, grown, and evaluated using PCR-based 16S ribosomal RNA (or 16S rRNA) nucleotide sequences. T. aphylla L., Melia azadirachta L., and Acacia arabica were the three crucial medicinal plants investigated to determine their antibacterial activity toward biofilm-forming strains. The findings showed that extracts of these three medicinal plants can be utilized against pathogenic bacteria that cause dental biofilms, and pharmaceutical companies should include them in dental care products [32]. The leaves of T. aphylla have been characterized as a rich source of tannins and flavonoids [50]. Kaempferol is a phytochemical found in T. aphylla. It has several therapeutic properties such as anticancer and antioxidant activities, while its usage is limited because of low permeability and poor solubility [78]. Kaempferol and resveratrol are two phenolic compounds that have shown antimicrobial activity. Therefore, researchers have employed two methods, time to kill and checkerboard, to evaluate this property. Studies showed that both compounds inhibited growth in MHB (Muller–Hilton broth) and milk according to the time-to-kill method [79]. In another study, T. aphylla suppressed the increase in microorganisms such as S. aureus and P. aeruginosa [64]. The possible antibacterial mechanism of T. aphylla is shown in Figure 4.
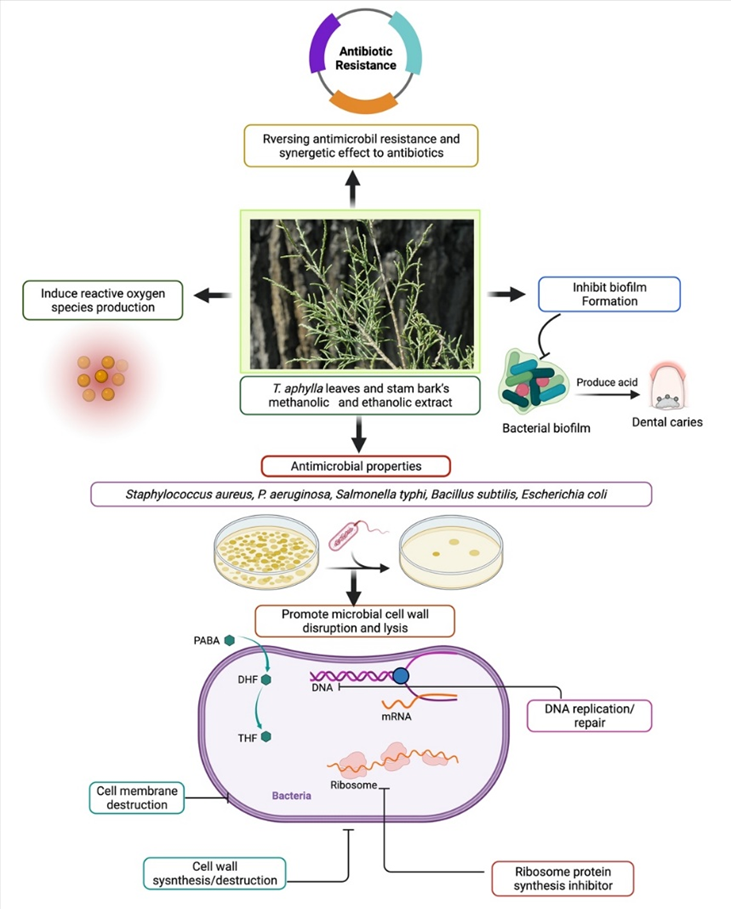
Figure 4. Possible antibacterial mechanism of T. aphylla against clinically important pathogens.
4.4. Antifungal Activity
Fungal infections are among the most dangerous diseases, killing around 1.5 million humans each year worldwide [80]. Infection of the skin ranks fourth among all fungal illnesses, and it also accounts for the bulk of deaths [81][82]. In the last several years, there has been an increase in the frequency of fungal illnesses and the resistance of specific fungus species to various fungicides used in medicine. Furthermore, fungi are among the most underappreciated diseases, while the reality is that amphotericin B and alternative commercially available antifungal therapies are still considered the gold standard. However, antifungal medicines have various side-effects such as toxicity, effectiveness, price, and extensive usage, resulting in resistant strains [83]. Resistance to antifungal medications has become a common issue because of their overuse. Therefore, plant-based therapies have piqued the interest of experts as a potential therapy for fungal infections [5]. The plant world has long been a hotbed for numerous natural chemicals with unique structures, which has piqued researchers’ curiosity in studying various plant species to this day. Numerous studies have shown that plants are a source of bioactive secondary metabolites, such as alkaloids, terpenoids, and saponins, all of which have demonstrated antifungal activities [84]. Traditional systems of medicine have also reported that medicinal herbs are fruitful candidates for treating animal and human mycoses. Furthermore, they can be used to develop novel antifungal drugs [83]. Accordingly, this section aims to explain the current situation regarding T. aphylla and its antifungal compounds, which may help to develop more effective antifungal medicines in the future.
A study was conducted to determine the effectiveness of ethanolic extracts of T. aphylla (2000 ppm, 1000 ppm, and 500 ppm) against six pathogenic fungi: Fusarium oxysporum, Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, Saccharomyces cerevisiae, and Penicillium notatum. Five different solvents (methanol, ethanol, acetone, chloroform, and distilled water) were used. The percentage suppression of fungus growth was shown to be dose-dependent. Terbinafine (synthetic drug) was administered in typical dosages with distilled water to test its effect against the various fungi. Terbinafine constrained the increase in A. flavus, A. fumigatus, A. niger, F. oxysporum, P. notatum, and S. cerevisiae at concentrations of 65 ± 0.58, 72 ± 1.00, 70 ± 1.15, 59 ± 1.00, 60 ± 0.58, and 80 ± 0.58 (µg/mL of PDA medium), respectively. The most effective solvent was chloroform, which prevented 97.68% ± 0.58% growth of F. oxysporum, 9.37% ± 0.33% growth of A. niger, 92.68% ± 3.33% growth of S. cerevisiae, 91.46% ± 2.08% growth of A. fumigatus, 88.48% ± 0.88% growth of A. flavus, and 87.95% ± 1.15% growth of P. notatum. Outcomes were statistically correlated to a negative control group, and most effects were highly significant (p ≤ 0.000). The stem–bark extract of T. aphylla showed the highest percentage of suppression with chloroform, followed by ethanol, acetone, methanol, and distilled water [85]. The methanolic extract of the bark of T. aphylla was examined in a study to counter bacterial pathogens in vitro to validate as Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Salmonella typhi, and fungal strains Candida albicans and Aspergillus flavus were tested using the disc diffusion assay with 25, 50, 75, and 100 mg/mL stock solutions prepared in dimethyl sulfoxide. The extract’s inhibitory impact in millimeters was calculated by measuring the zone of growth inhibition surrounding the discs and comparing it to the reference medication. T. aphylla bark established a marked zone of inhibition at higher concentration for most fungal and bacterial strains. According to the findings, T. aphylla produces bioactive and phytochemical substances used in healthcare [76]. However, there is a scarcity of research and comprehensive reviews on T. aphylla as an alternative to antifungal medications. There is a need to study the structure–activity relationship, molecular mechanism, and potential synergistic effects of components of T. aphylla. These findings suggest the need for further research on T. aphylla to manage antifungal diseases.
4.5. Periodontal Disease
After dental caries, periodontitis is the second most prevalent dental disease worldwide [86][87]. It is caused by forming an organized biofilm of bacterial strain, so-called dental plaque, in the subgingival area, affixed to host tissues and cells. This ultimately results in loss of supporting tooth structure [88][89]. The most common cause of periodontal diseases is infection and inflammation of the alveolar bone and gingiva, which supports the tooth in the socket [90]. Gingivitis is a disease of the gums in which they become red and swollen with a high bleed tendency without loss of supporting tooth structure [91][92][93]. Adults are more likely to get periodontal disease, mainly slow and moderately progressive periodontitis. Biofilms are multispecies bacterial colonies that invade the oral cavity as plaque, causing dental cavities and periodontal disease [94]. In a study, three herbs were examined to determine the effectiveness against pinpointed dental biofilm-forming bacteria. Pathogenic strains that form dental biofilm were isolated, grown, and evaluated using PCR-based 16S ribosomal RNA (or 16S rRNA) nucleotide sequences [32].
T. aphylla, Melia azadirachta, and Acacia arabica were the three crucial medicinal plants investigated to determine their antibacterial activity against biofilm-forming strains. The findings showed that extracts of these three medicinal plants can be utilized against pathogenic bacteria that cause dental biofilms. Phylogenetic examinations explored 19 strains linked to Firmicutes, Actinobacteria, and Proteobacteria. Eleven of the 19 isolates were discovered to have a strong biofilm-forming potential, and an antibiotic activity assay revealed that the chosen herbs suppressed their development. Therefore, the findings showed that extracts of these medicinal plants can be utilized to guard against pathogenic bacteria that cause dental biofilms. Therefore, pharmaceutical companies should include them in dental care products [32]. Periodontal disease and the probable role of T. aphylla are illustrated in Figure 5.
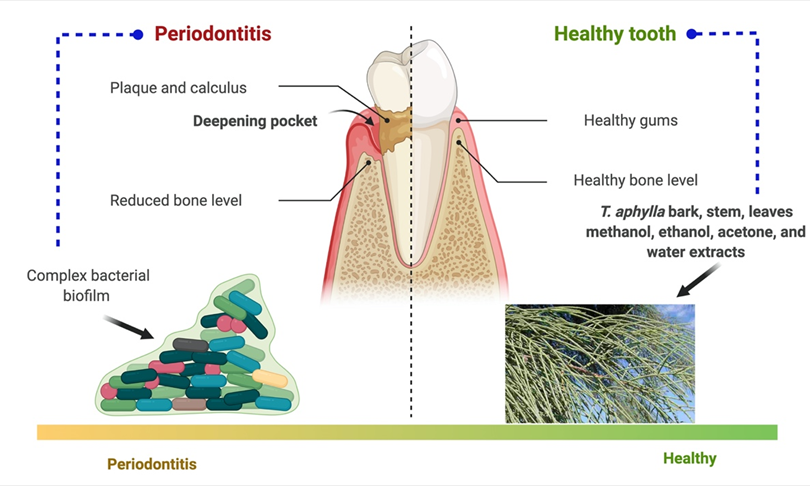
Figure 5. Periodontal disease and the probable role of T. aphylla.
References
- Narendhirakannan, R.T.; Subramanian, S.; Kandaswamy, M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1150–1157.
- Hussain, A.; Ahmad, M.; Wahab, S.; Hussain, M.; Ali, M. A review on pharmacological and phytochemical profile of Asparagus racemosus Willd. Pharmacologyonline 2011, 3, 1353–1364.
- Alsayari, A.; Muhsinah, A.B.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological efficacy of ginseng against respiratory tract infections. Molecules 2021, 26, 4095.
- Ali, M.; Alhazmi, H.A.; Ansari, S.H.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and Bioactive Profile Compilations of Less Discussed but Effective Naturally Growing Saudi Plant. In Plant and Human Health, Volume 3; Springer: Cham, Switzerland, 2019; Volume 3, pp. 343–352. ISBN 9783030044084.
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404.
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza glabra in the treatment of respiratory tract infections. Mini-Rev. Med. Chem. 2021, 21.
- Pradeepa, S.; Subramanian, S.; Kaviyarasan, V. Biochemical evaluation of antidiabetic properties of Pithecellobium dulce fruits studied in streptozotocin induced experimental diabetic rats. Int. J. Herb. Med. 2013, 1, 21–28.
- Ullah, R.; Tariq, S.A.; Khan, N.; Sharif, N.; Ud Din, Z.; Mansoor, K. Antihyperglycemic effect of methanol extract of Tamarix aphylla L. Karst (Saltcedar) in streptozocin–nicotinamide induced diabetic rats. Asian Pac. J. Trop. Biomed. 2017, 7, 619–623.
- Gaskin, J.F. Tamaricaceae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2003; pp. 363–368.
- Baum, B.R. The Genus Tamarix.—Jerusalem: The Israel Academy of Sciences and Humanities; Publications Jerusalem; Israel Academy of Sciences and Humanities: West Jerusalem, Israel, 1978.
- Alnuqaydan, A.M.; Rah, B. Tamarix articulata (T. articulata)—An Important Halophytic Medicinal Plant with Potential Pharmacological Properties. Curr. Pharm. Biotechnol. 2019, 20, 285–292.
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245.
- Samadi, N.; Ghaffari, S.M.; Akhani, H. Meiotic behaviour, karyotype analyses and pollen viability in species of Tamarix (Tamaricaceae). Willdenowia 2013, 43, 195–203.
- Mustafa Akhlaq, A.M. New phenolic acids from the galls of Tamarix aphylla (L.) Karst. Int. Res. J. Pharm. 2011, 2, 222–225.
- Barakat, H.H.; Nada, S.A. Chemical and Biological Investigations of the Constitutive Phenolics of Two Egyptian Folk-Medicinal Plants; A Novel Phenolic from the Galls of Tamarix aphylla. Nat. Prod. Sci. 1996, 2, 96–101.
- Nawwar, M.A.; Ayoub, N.A.; El-Rai, M.A.; Bassyouny, F.; Mostafa, E.S.; Al-Abd, A.M.; Harms, M.; Wende, K.; Lindequist, U.; Linscheid, M.W. Cytotoxic ellagitannins from Reaumuria vermiculata. Fitoterapia 2012, 83, 1256–1266.
- Mahfoudhi, A.; Prencipe, F.P.; Mighri, Z.; Pellati, F. Metabolite profiling of polyphenols in the tunisian plant tamarix aphylla (L.) Karst. J. Pharm. Biomed. Anal. 2014, 99, 97–105.
- Nawwar, M.A.M.M.; Hussein, S.A.M.M.; Ayoub, N.A.; Hofmann, K.; Linscheid, M.; Harms, M.; Wende, K.; Lindequist, U. Aphyllin, the first isoferulic acid glycoside and other phenolics from Tamarix aphylla flowers. ChemInform 2009, 40, 342–347.
- Majumder, P.; Paridhavi, M. A comprehensive ethno-phyto-pharmacological review on novel Indian medicinal plants used in polyherbal formulations. Int. J. Phytomed. 2013, 5, 394–414.
- Ahmad, M.; Zafar, M.; Sultana, S. Salvadora persica, Tamarix aphylla and Zizyphus mauritiana-Three Woody Plant Species Mentioned in Holy Quran and Ahadith and Their Ethnobotanical Uses in North Western Part (D.I. Khan) of Pakistan. Pak. J. Nutr. 2009, 8, 542–547.
- Qadir, M.I.; Abbas, K.; Hamayun, R.; Ali, M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L. (Saltcedar) in mice. Pak. J. Pharm. Sci. 2014, 27, 1985–1988.
- Laaroussi, I. Natural Product Temporarily Tightening the Mucous Membranes of the Vagina. Patent Application No. WO/2012/052627, 26 April 2012.
- Marwat, S.K.; Rehman, F.U.; Khan, M.A.; Ahmad, M.; Zafar, M.; Ghulam, S. Medicinal folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. Pak. J. Bot. 2011, 43, 1453–1462.
- Ullah, S.; Rashid Khan, M.; Ali Shah, N.; Afzal Shah, S.; Majid, M.; Asad Farooq, M. Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. J. Ethnopharmacol. 2014, 158, 412–422.
- Zhang, D.; Yin, L.; Pan, B. Biological and ecological characteristics ofTamarix L. and its effect on the ecological environment. Sci. China Ser. D Earth Sci. 2002, 45, 18–22.
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117.
- Al-Fatimi, M. Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. J. Ethnopharmacol. 2019, 241, 111973.
- Shah, A.; Rahim, S.; Bhatti, K.H.; Khan, A.; Din, N.; Imran, M.; Mohsin, M.; Ishtiaq, M.; Nabila, A.; Ansari, A.; et al. Ethnobotanical study and conservation status of trees in the district Sargodha, Punjab, Pakistan. Phyton 2015, 84, 34–44.
- Bahadur, S.; Khan, M.S.; Shah, M.; Shuaib, M.; Ahmad, M.; Zafar, M.; Begum, N.; Gul, S.; Ashfaq, S.; Mujahid, I.; et al. Traditional usage of medicinal plants among the local communities of Peshawar valley, Pakistan. Acta Ecol. Sin. 2020, 40, 1–29.
- Saeed Khattak, N.; Nouroz, F.; Ur Rahman, I.; Noreen, S. Ethno veterinary uses of medicinal plants of district Karak, Pakistan. J. Ethnopharmacol. 2015, 171, 273–279.
- Yaseen, G.; Ahmad, M.; Zafar, M.; Sultana, S.; Kayani, S.; Cetto, A.A.; Shaheen, S. Traditional management of diabetes in Pakistan: Ethnobotanical investigation from Traditional Health Practitioners. J. Ethnopharmacol. 2015, 174, 91–117.
- Khalid, M.; Hassani, D.; Bilal, M.; Butt, Z.A.; Hamayun, M.; Ahmad, A.; Huang, D.; Hussain, A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral Biol. 2017, 81, 175–185.
- Umair, M.; Altaf, M.; Bussmann, R.W.; Abbasi, A.M. Ethnomedicinal uses of the local flora in Chenab riverine area, Punjab province Pakistan. J. Ethnobiol. Ethnomed. 2019, 15, 7.
- Uzair, M.; Ijaz, A.S.; Khan, T.R. Survey of Ethno-Medicinal Weeds of District Rajhan Pur. Indian Res. J. Pharm. Sci. 2014, 1, 38–45.
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367.
- Alzweiri, M.; Al Sarhan, A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35.
- Suleiman, M.H.A. Ethnobotanical, Phytochemical, and Biological Study of Tamarix aphylla and Aerva javanica Medicinal Plants Growing in the Asir Region, Saudi Arabia. Trop. Conserv. Sci. 2019, 12, 1940082919869480.
- Kamal, M.; Wazir, S.M.; Hassan, M.; Saad, M.S.; Khan, U.; Muhammad, A.; Taj, S. Ethnobotanically Important Plants of District Bannu, Pakistan. J. Plant Sci. 2009, 15, 87–93.
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.-L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103.
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 2000, 71, 278–307.
- Pittler, M. A Guide to Medicinal Plants. Focus Altern. Complementary Ther. 2010, 14, 354.
- Brock, J.H. Tamarix spp. (Salt Cedar), an invasive exotic woody plant in arid and semi-arid riparian habitats of western USA. Ecol. Manag. Invasive Riverside Plants 1994, 4, 27–44.
- Horton, J.S. Notes on the Introduction of Deciduous Tamarisk. Available online: https://gifiloqokawom.pdfleadership.icu/notes-on-the-introduction-of-deciduous-tamarisk-book-21438vb.php (accessed on 9 August 2021).
- Jasiem, T.M.; Nasser, N.M.; Al-Bazaz, H.K. Tamarix aphylla L.: A review. Res. J. Pharm. Technol. 2019, 12, 3219–3222.
- Nawwar, M.A.M.; Hussein, S.A.M. Gall polyphenolics of Tamarix aphylla. Phytochemistry 1994, 36, 1035–1037.
- Nag, G.; De, B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int. J. Pharm. Pharm. Sci. 2011, 3, 121–124.
- Sharma, S.K.; Parmar, V.S.; Shanna, S.K.; Parmar, V.S. Novel constituents of Tamarix species. J. Sci. Ind. Res. 1998, 57, 873–890.
- Souliman, A.M.A.; Barakat, H.H.; El-Mousallamy, A.M.D.; Marzouk, M.S.A.; Nawwar, M.A.M. Phenolics from the bark ofTamarix aphylla. Phytochemistry 1991, 30, 3763–3766.
- Nawwar, M.A.M.; Hussein, S.A.M.; Buddrus, J.; Linscheid, M. Tamarixellagic acid, an ellagitannin from the galls of Tamarix aphylla. Phytochemistry 1994, 35, 1349–1354.
- Shafaghat, A. Phytochemical investigation of quranic fruits and plants. J. Med. Plants 2010, 9, 61–66.
- Orabi, M.A.A.; Taniguchi, S.; Sakagami, H.; Yoshimura, M.; Yoshida, T.; Hatano, T. Hydrolyzable tannins of tamaricaceous plants. V. Structures of monomeric-trimeric tannins and cytotoxicity of macrocyclic-type tannins isolated from tamarix nilotica (1). J. Nat. Prod. 2013, 76, 947–956.
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus—Biol. 2008, 331, 865–873.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218.
- Wahab, S.; Ahmad, M.F.; Hussain, A.; Usmani, S.; Shoaib, A.; Ahmad, W. Effectiveness of Azithromycin as add-on Therapy in COVID-19 Management. Mini-Rev. Med. Chem. 2021, 21, 2860–2873.
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315.
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1.
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143.
- Gadallah, A.S.; Mujeeb-Ur-Rehman; Atta-Ur-Rahman; Yousuf, S.; Atia-Tul-Waha; Jabeen, A.; Swilam, M.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Iqbal Choudhary, M. Anti-inflammatory principles from Tamarix aphylla L.: A bioassay-guided fractionation study. Molecules 2020, 25, 2994.
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914.
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229.
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490.
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229.
- Abbas, B.; Al-Qarawi, A.A.; Al-Hawas, A. The ethnoveterinary knowledge and practice of traditional healers in Qassim region, Saudi Arabia. J. Arid. Environ. 2002, 50, 367–379.
- Sajid Ali, M.; Sarfaraz Alam, M.; Ahmad, S.; Ali, M.; Ahsan, W.; Siddiqui, M.R.; Salahuddin Ansari, M.; Shamim, M.; Ali, M.D. Wound healing activity of alcoholic extract of Tamarixaphylla L. On animal models. Biomed. Pharmacol. J. 2019, 12, 41–48.
- Soliman Yu, H.; Ibrahim Al, S. Anti-inflammatory and Wound Healing Activities of Herbal Gel Containing an Antioxidant Tamarix aphylla Leaf Extract. Int. J. Pharmacol. 2011, 7, 829–835.
- Hussain, A.; Rizvi, A.; Wahab, S.; Ansari, S.; Hussain, S.; Zareen, I. Antibacterial Screening of the Bark of Adenanthera pavonina (L.). Int. J. Biomed. Res. 2011, 2, 110–122.
- Hussain, A.; Wahab, S.; Zarin, I.; Hussain, M.D.S. Antibacterial Activity of the Leaves of Coccinia indica (W. and A) Wof India. Biol. Res. 2010, 4, 241–248.
- Ahmad, M.D.F.; Wahab, S.; Ali Ahmad, F.; Intakhab Alam, M.; Ather, H.; Siddiqua, A.; Amir Ashraf, S.; Abu Shaphe, M.; Idreesh Khan, M.; Ali Beg, R. A novel perspective approach to explore pros and cons of face mask in prevention the spread of SARS-CoV-2 and other pathogens. Saudi Pharm. J. 2021, 29, 121–133.
- El-Zayat, M.M.; Eraqi, M.M.; Alfaiz, F.A.; Elshaer, M.M. Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine. J. King Saud Univ.-Sci. 2021, 33, 101466.
- Verma, R.; Pavithra, P.; Janani, V.; Charumathi, K.; Indumathy, R.; Potala, S. Antibacterial activity of plants used in Indian herbal medicine. Int. J. Green Pharm. 2010, 4, 22.
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316.
- Adnan, M.; Tariq, A.; Bibi, R.; AbdElsalam, N.; Rehman, H.; Murad, W.; Ahmad, S.; Israr, M.; Sabahat, S.; Ullah, R.; et al. Antimicrobial potential of alkaloids and flavonoids extracted from Tamarix aphylla leaves against common human pathogenic bacteria. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 27.
- Taghipour, M.T.; Nameni, R.; Taghipour, M.; Ghorat, F. Phytochemical Analysis and Antimicrobial Activity of Ziziphus spina-christi and Tamarix aphylla Leaves’ Extracts as Effective Treatment for Coronavirus Disease 2019 (COVID-19). Thrita 2020, 9, e107776.
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262.
- Al Sobeai, S.M. Anticancer, Cytotoxic Effect of Tamarix Aphylla, and Antibacterial Screening Efficiency against Multidrug-Resistant Human Pathogens. Asian J. Pharm. Clin. Res. 2018, 11, 241.
- Iqbal, H.; Ishfaq, M.; Abbas, M.N.; Ahmad, I.; Rehman, A.; Amin, S.B.; Shagufta, B.I.; Ullah, M. In vitro Antimicrobial Study of Tamarix aphylla in View of Phytochemical Constituents. Pharmacologia 2015, 6, 333–336.
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75.
- Diao, M.; Liang, Y.; Zhao, J.; Zhao, C.; Zhang, J.; Zhang, T. Enhanced cytotoxicity and antioxidant capacity of kaempferol complexed with α-lactalbumin. Food Chem. Toxicol. 2021, 153, 112265.
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríquez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220.
- Sanglard, D. Clinical relevance of mechanisms of antifungal drug resistance in yeasts. Enferm. Infecc. Microbiol. Clínica 2002, 20, 462–470.
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534.
- Ghazwani, M.; Hani, U.; Osmani, R.A.M.; Rahamathulla, M.; Begum, M.Y.; Wahab, S.; Gowda, D.V.; Ravikumar, A.A.; Kumar, H.Y.; Ather, H.; et al. An Efficient Herbal Approach for Treating Fungal Infection in Cervical Cancer Patients by Developing and Optimizing a Vaginal Suppository. Int. J. Polym. Sci. 2021, 2021, 9198387.
- Kumar Mishra, K.; Deep Kaur, C.; Kumar Sahu, A.; Panik, R.; Kashyap, P.; Prasad Mishra, S.; Dutta, S. Medicinal Plants Having Antifungal Properties. In Medicinal Plants—Use in Prevention and Treatment of Diseases ; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78985-888-4.
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638.
- Bibi, S.; Afzal, M.; Khan, M.B. Antifungal Activity of Tamarix aphylla (L.) Karst. Stem-bark Extract Against Some Pathogenic Fungi. Am.-Eurasian J. Agric. Environ. Sci. 2015, 5, 44–48.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8.
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal disease and its prevention, by traditional and new avenues (Review). Exp. Ther. Med. 2019, 19, 1504.
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444.
- Ahmad, I.; Wahab, S.; Nisar, N.; Dera, A.A.; Alshahrani, M.Y.; Abullias, S.S.; Irfan, S.; Alam, M.M.; Srivastava, S. Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J. 2020, 28, 1203–1209.
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135.
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114.
- Orban, B. Gingivitis. J. Periodontol. 1955, 26, 173–179.
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S44–S67.
- Chen, L.H. (Ed.) Nutritional Aspects of Aging; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351075145.
More
Information
Subjects:
Plant Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




