Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giulia Gorrieri | + 2248 word(s) | 2248 | 2022-02-10 07:32:38 | | | |
| 2 | Camila Xu | -37 word(s) | 2211 | 2022-02-17 02:15:18 | | | | |
| 3 | Camila Xu | + 49 word(s) | 2260 | 2022-02-18 09:47:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gorrieri, G. SLC26A9 in Cystic Fibrosis Lung Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/19532 (accessed on 07 February 2026).
Gorrieri G. SLC26A9 in Cystic Fibrosis Lung Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/19532. Accessed February 07, 2026.
Gorrieri, Giulia. "SLC26A9 in Cystic Fibrosis Lung Disease" Encyclopedia, https://encyclopedia.pub/entry/19532 (accessed February 07, 2026).
Gorrieri, G. (2022, February 16). SLC26A9 in Cystic Fibrosis Lung Disease. In Encyclopedia. https://encyclopedia.pub/entry/19532
Gorrieri, Giulia. "SLC26A9 in Cystic Fibrosis Lung Disease." Encyclopedia. Web. 16 February, 2022.
Copy Citation
SLC26A9 belongs to the solute carrier family 26 (SLC26), which comprises membrane proteins related to the phylogenetically older SLC26-SulP gene family. On the basis of different preliminary findings, including the phenotype of SlC26A9-deficient mice and its possible role as a gene modifier of the human phenotype and treatment response, SLC26A9 has emerged as one of the most interesting alternative targets for the treatment of cystic fibrosis (CF).
SLC26A9
cystic fibrosis
gene modifiers
anion transport
1. Introduction
SLC26A9 belongs to the solute carrier family 26 (SLC26), which comprises membrane proteins related to the phylogenetically older SLC26-SulP gene family. As part of the APC superfamily, such proteins operate as electroneutral or electrogenic exchangers of monovalent and divalent anions and as anion channels [1]. The mammalian SLC26 family includes 11 genes (SLC26A1-A11) expressed throughout the body, with a heterogeneous tissue distribution and a variety of functions within a conserved molecular scaffold [1][2]. Three members of the family have been identified as disease-causing genes in different human genetic disorders: SLC26A2 in chondrodysplasias [3], SLC26A3 in chloride-losing diarrhea [4], and SLC26A4 in Pendred syndrome and hereditary deafness (DFNB4) [5][6][7]. Additionally, based on their possible roles in anion transport, some members of the family, including SLC26A4 and SLC26A9, have been linked to cystic fibrosis (CF) as disease modifiers or potential therapeutic targets. CF, which is one of the most common monogenic diseases and affects at least 100,000 individuals worldwide, is caused by mutations in the gene encoding the CFTR anion channel [8]. Although CF is a multi-organ disease, the most severe manifestations are observed in the lung, where the loss of the chloride and bicarbonate secretion mediated by CFTR dehydrates and acidifies the thin layer of fluid covering the airways and impairs the mucociliary clearance and the innate immunity, thus resulting in chronic infection, inflammation, and progressive structural and functional lung damage [9][10][11][12][13][14][15].
2. SLC26A9 Tissue, Cellular, and Subcellular Localization
2.1. SLC26A9 Expression in Human
The original description of SLC26A9 as a lung-expressed protein drew attention to SLC26A9 regarding its possible role in airway physiology and disease [16]. However, following studies focusing on SLC26A9 rarely managed to conclusively confirm and extend such an observation. Figure 1 and Table 1 summarize the major findings regarding SLC26A9 expression in tissues, primary cells, and immortalized cell lines of human origins at the mRNA and protein levels.
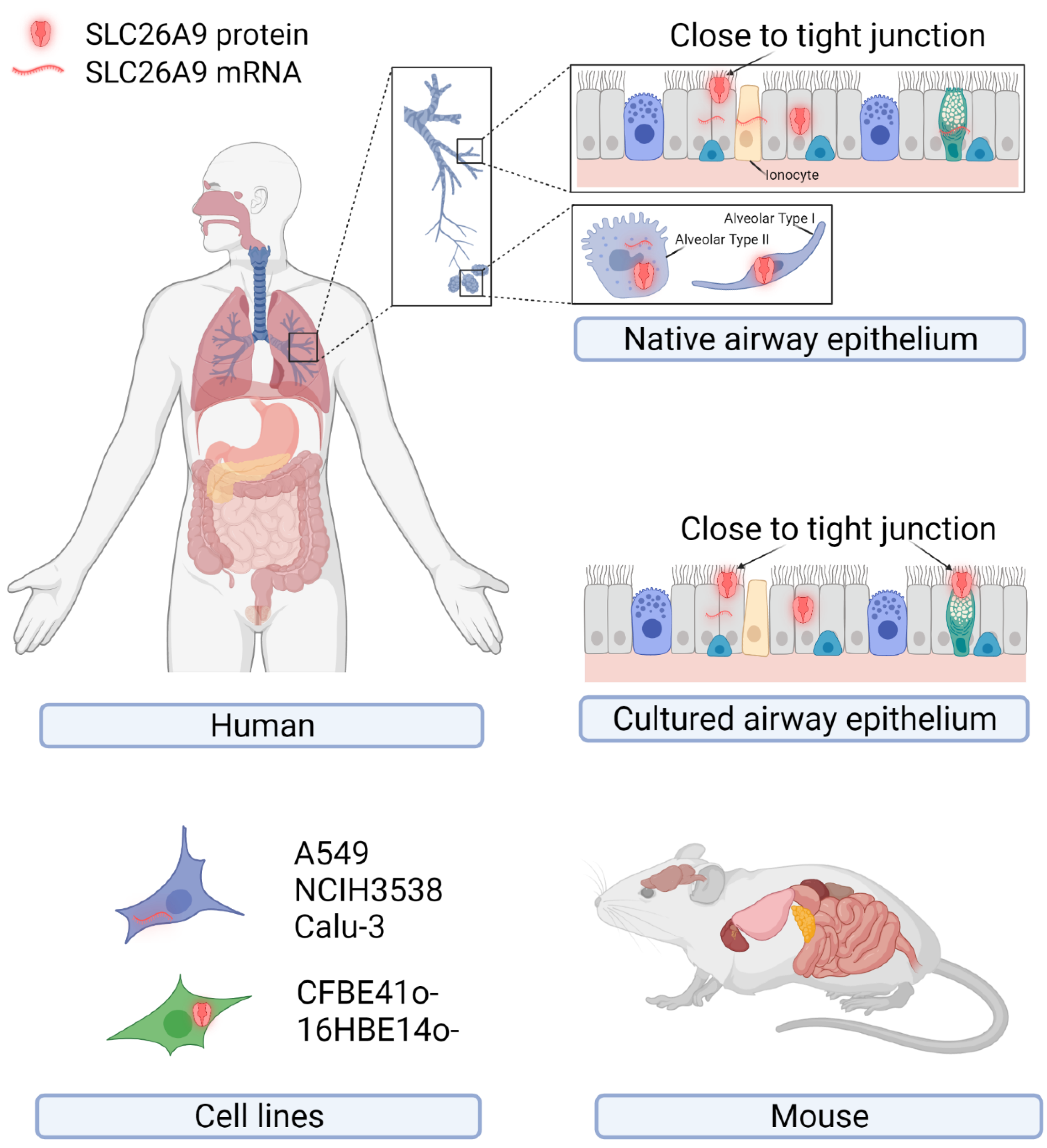 Figure 1. Endogenous expression of SLC26A9 in human tissues, primary and immortalized cell lines of human origin, and mouse tissues. The cartoons show tissues and cells reported to express SLC26A9 at the mRNA and/or protein levels. Details of the putative subcellular localization (intracellular compartments or plasma membrane close to the tight junctions) are shown for the native and cultured airway epithelia. Created with BioRender.com (21 January 2022).
Figure 1. Endogenous expression of SLC26A9 in human tissues, primary and immortalized cell lines of human origin, and mouse tissues. The cartoons show tissues and cells reported to express SLC26A9 at the mRNA and/or protein levels. Details of the putative subcellular localization (intracellular compartments or plasma membrane close to the tight junctions) are shown for the native and cultured airway epithelia. Created with BioRender.com (21 January 2022).Table 1. Endogenous expression of the human SLC26A9. Data from the literature are listed as: “detection method: sample showing expression”. IHC: immunohistochemistry; IF: immunofluorescence.
| Tissues | Primary Cells | Cell lines | References | |||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | |
| RT-PCR, Northern blot: lung, pancreas, prostate | IHC: lung bronchial and alveolar cells | - | - | RT-PCR: NCIH3538, A549 | - | [16] |
| - | - | RT-PCR: CF and non-CF bronchial epithelial cells | - | - | - | [17] |
| real-time PCR: GI tract (mostly stomach) | - | - | - | - | - | [18] |
| - | - | qRT-PCR: non-CF nasal cells, CF, and non-CF bronchial epithelial cells | - | qRT-PCR: Calu-3 | - | [19] |
| - | - | TaqMan®SNP Genotyping system: nasal cells | - | - | - | [20] |
| - | - | - | IF: non-CF bronchial epithelial cells (goblet and ciliated cells) | - | - | [21] |
| scRNA-Seq: pancreas (ductal and ductar/acinar cells) | - | - | - | - | - | [22] |
| - | - | sqRT-PCR: CF and non-CF bronchial epithelial cells | - | - | - | [23] |
| IF: CF and non-CF lung tissues | - | - | Western blot: nasal epithelial cells | - | Western blot: Immortalized human bronchial epithelial cells–16HBE14o-, CFBE41o- | [24] |
As mentioned above, the first observation by Lohi and colleagues detected SLC26A9 by immunohistochemistry with a self-produced antibody (directed against the peptide “ELSLYDSEEDIRSYWD-LEQE”, corresponding to amino acids 758–777 of the human SLC26A9; see Figure 2) on archival specimens of adult human kidney, testis, and lung. SLC26A9 was found to be expressed in the cytoplasm of bronchial and alveolar epithelial cells (Figure 1) [16]. In the same work, SLC26A9 was found endogenously expressed, at the mRNA level, in NCI-H3538 and A549 cell lines (derived from bronchoalveolus and alveolus, respectively) [16].
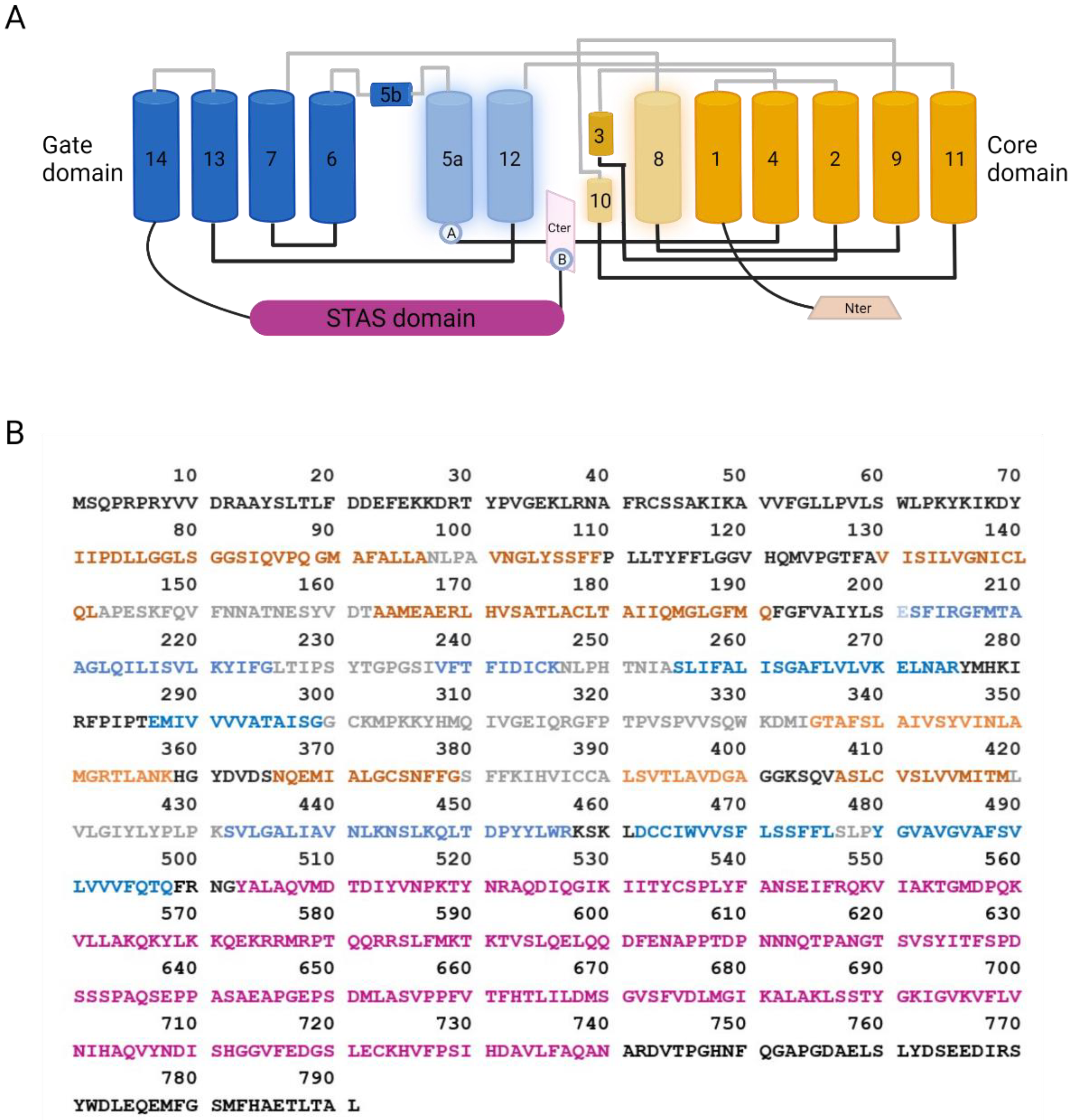 Figure 2. SLC26A9 structure. (A) Cartoon showing the topology of SLC26A9, as determined by Chi and colleagues. The gate domain is colored in blue, the core domain is colored in orange, and their participation in the intracellular pocket is highlighted by increased transparency. Extracellular and intracellular loops are colored in grey and black, respectively. The STAS domain is colored in purple, C-terminal in pink, and N-terminal in light orange. Ⓐ: Location of E201K mutation in TM5, which, together with the C-terminal mutation S781A, provided increased currents measured by single-channel recordings. The same results were obtained by mutating different residues visually represented by Ⓑ: E775A/Q776A/F779G/S781G/M782G/F783G and E775A/Q776A. Those mutations can interfere with the interaction between the C-terminal and the TM5 or TM12 [25]. Created with BioRender.com (21 January 2022); (B) Amino acid sequence of SLC26A9. Residues are colored as the corresponding domains in (A).
Figure 2. SLC26A9 structure. (A) Cartoon showing the topology of SLC26A9, as determined by Chi and colleagues. The gate domain is colored in blue, the core domain is colored in orange, and their participation in the intracellular pocket is highlighted by increased transparency. Extracellular and intracellular loops are colored in grey and black, respectively. The STAS domain is colored in purple, C-terminal in pink, and N-terminal in light orange. Ⓐ: Location of E201K mutation in TM5, which, together with the C-terminal mutation S781A, provided increased currents measured by single-channel recordings. The same results were obtained by mutating different residues visually represented by Ⓑ: E775A/Q776A/F779G/S781G/M782G/F783G and E775A/Q776A. Those mutations can interfere with the interaction between the C-terminal and the TM5 or TM12 [25]. Created with BioRender.com (21 January 2022); (B) Amino acid sequence of SLC26A9. Residues are colored as the corresponding domains in (A).Besides these findings, not much more effort has been made in the characterization of SLC26A9 protein expression in human airway tissue, until the very recent work by Pinto and colleagues [24]. SLC26A9 appeared localized in the apical pole of bronchial epithelial cells, possibly overlapping CFTR and the tight junction marker ZO-1, in control tissues, whereas showed cytoplasmic staining in tissues from CF donors carrying F508del-CFTR [24].
Various studies have investigated SLC26A9 expression in human tissues at the mRNA level by RT-PCR, Northern blotting, and scRNA-Seq, indicating the lung, the pancreas (ductal and acinar cells), the prostate, and the stomach (fundus/corpus and antrum region) as the main sites of expression, whereas lower levels have been found in the proximal duodenum (Figure 1) [16][18][22]. Regarding the airway tissues, recent results provided by scRNA-Seq studies seem to indicate a very low expression in airway epithelial cells of the upper airways, including ciliates cells, club cells, and ionocytes, whereas a higher level has been detected in alveolar cells (particularly type 2) [26].
Given its possible role in CF, SLC26A9 expression has been investigated in epithelial cells collected from CF and non-CF individuals and cultured under air–liquid conditions. Different research teams have provided evidence of some SLC26A9 expression at the mRNA level [20][17][19][23]. Sato and colleagues also detected SLC26A9 by immunofluorescence (with the NBP2-30425 antibody) on well-differentiated bronchial epithelia, localizing it at the apical membrane periphery, close to the tight junctions of goblet and ciliated cells [21]. Interestingly, the disruption of the tight junction complexes by the calcium chelating agent EGTA caused both ZO-1 and SLC26A9 immunofluorescence signals to become more diffuse [21].
Concerning the subcellular localization, additional and sometimes controversial data come from studies relying on the heterologous expression of SLC26A9 in Xenopus oocytes and various immortalized cell lines. The transfection of the full-length coding sequence in HEK-293 cells resulted in a high rate of protein expression with the accumulation of intracellular aggregates and almost an absence of SLC26A9 protein at the plasma membrane [27][25]. Retention in intracellular compartments was also the main behavior found in FRT-transfected cells, with only a minor fraction of the protein apparently able to traffic to the cell periphery [28].
2.2. SLC26A9 Expression in Mouse
At the mRNA level, high expression has been detected by RT-PCR and Northern blotting in the stomach, trachea, lung, and duodenum crypt [29][18][30]. Lower levels have been found in the brain, heart, kidney, thymus, spleen, and ovary [31]. In situ hybridization revealed abundant expression in the surface epithelial cells of the stomach [30]. Slc26a9 protein was detected by immunofluorescence labeling by different authors. By using a self-produced anti-Slc26a9 antibody (directed against the mouse synthetic peptide “CDTEFSLYDSEEEGP”, corresponding to human residues 755–778 in Figure 2), a positive signal was found in stomach surface epithelial cells with apical localization and in the body of gastric glands [30]. Similarly, with a second self-produced antibody (directed against the C-terminus sequence “CKQKYLRKQEKRTAIPTQQRK”, corresponding to human residues 565–584), Slc26a9 was found localized in the lung (bronchial epithelial and alveolar cells) and stomach (apical membrane of gastric surface epithelia and intracellular membranes) [31]. Slc26a9 was also found at the apical membrane of principal cells in the medullary collecting duct of the kidney [32].
3. SLC26A9 Protein Structure and Function
3.1. SLC26A9 Structure
Basic features of the SLC26A9 structure have been initially deduced from the crystal structure of the bacterial homolog SLC26Dg, showing a transmembrane domain (TM) folding such as UraA and NBCe1 [2]. Subsequently, Walter and colleagues gained insight into the SLC26A9 structure by the cryo-electron microscopy (cryo-EM) of a truncated version of the mouse protein [27]. Indeed, in order to improve protein purification, the authors designed a minimal construct where two predicted intrinsically disordered regions were removed from the C-terminal part of the protein (residues P558-V660 of STAS domain and the final 44 residues of the C-terminus P745-L790 containing a PDZ motif) [27]. Such modifications, besides increasing the protein purification rate, also resulted in the higher plasma membrane localization of the truncated SLC26A9 with respect to the full-length murine construct [27]. More recently, Chi and colleagues were able to resolve, again by cryo-EM, the molecular structure of the full-length human protein at an overall resolution of 2.6 Å [25].
SLC26A9 exists as a homodimer. Each protomer can be divided into a TM domain and a cytosolic domain composed, respectively, of 14 transmembrane segments and the N- and C-terminal tails of the protein. As modeled in Figure 2, the small N-terminus connects with the first of 14 transmembrane helices, whereas the C-terminus contains a PDZ domain, and a Sulfate Transporter and Anti-Sigma factor antagonist (STAS) domain linked to TM14. Within the TM domain, different helices assemble to form the flanking core (TM1–4 and TM8–11) and gate (TM5–7 and TM12–14) domains.
3.2. SLC26A9 Transport Properties
The transport properties of SLC26A9 have been investigated by means of various experimental approaches. SLC26A9 was found to be permeable to SO₄²-, Cl−, and C₂O₄²− by isotope uptake assays in Xenopus oocytes expressing the human protein [16]. Intracellular pH measurements with the BCECF probe in HEK-293-transfected cells found SLC26A9 mediating Cl−/HCO3− exchange and Cl−-independent HCO3− extrusion [30]. Subsequently, by isotope uptake and patch-clamp recording in oocytes, HEK-293, and CHO cells, mouse Slc26a9 was associated with three ion transport modes: electrogenic nCl−/HCO3− exchange, electrogenic Na+/nAnion− cotransport, and anion channel [31].
3.3. SLC26A9 Interaction with CFTR
Several studies have investigated the physical and functional interaction between SLC26A9 and CFTR.
A possible direct interaction between SLC26A9 and CFTR, involving the STAS domain of SLC26A9 and the regulatory domain of CFTR, was depicted in Xenopus oocytes and resulted in the inhibition of SLC26A9 activity [33]. Many other studies investigated the functional outcomes of SLC26A9 and CFTR co-expression.
Concerning CFTR activity, the co-transfection of SLC26A9 and wt-CFTR in HEK-293 cells resulted in enhanced forskolin-stimulated CFTR-dependent current [17]. Importantly, in immortalized human bronchial epithelial cell lines (CFBE41o− and 16HBE14o−), SLC26A9 overexpression and silencing resulted in increased and reduced CFTR expression and function, respectively [24].
Concerning SLC26A9 activity, some studies reported a low contribution to anion transport, either in the presence or in the absence of CFTR [34][35]. Rather, SLC26A9 transfection in HEK-293 resulted in the appearance of constitutive anion currents, both if expressed alone or in case of co-expression with wt-CFTR [17]. A similar behavior was observed with the co-expression of G551D-CFTR, a gating mutation with a normal expression at the plasma membrane [28][36]. Instead, if the co-expression occurred with F508del-CFTR, a trafficking mutation causing endoplasmic-reticulum retention, a noteworthy reduction in SLC26A9 activity was shown in three different models (HEK-293 and BHK cells, and Xenopus laevis oocytes) [21][36][34]. Such findings suggest that the two proteins interact early during their intracellular maturation. Therefore, the presence of the trafficking-incompetent F508del-CFTR may prevent SLC26A9 from moving to the cell membrane. This hypothesis was supported by coimmunoprecipitation assays, where SLC26A9 coimmunoprecipitated with both the mature and immature CFTR [36].
3.4. SLC26A9 Contribution to Epithelial Ion Transport
The contribution of SLC26A9 to transepithelial anion secretion has been investigated in different epithelial models. The heterologous expression of SLC26A9 in FRT cells cultured on porous supports increased the baseline anion current measured by short-circuit current recordings [28]. Such activity was abolished under the Cl−-free condition and was inhibited by GlyH-101, niflumic acid, and DIDS (which are all commonly used wide-spectrum anion channel blockers), but not by a specific CFTR inhibitor, CFTRinh-172 [17][28]. A constitutive anion secretion not obviously ascribable to other channels, and inhibited by GlyH-101, was also observed in some studies on human bronchial epithelia [17][36]. This constitutive current was affected by tight junction disruption and was absent in bronchial epithelia from F508del homozygous patients, both conditions reported to reduce SLC26A9 expression at the plasma membrane [21][36]. These findings suggest SLC26A9 as the responsible molecular identity, although conclusive evidence is lacking. Moreover, the contribution of such potential SLC26A9-dependent anion secretion to epithelial physiology and disease still needs to be investigated.
In mice airways, SLC26A9 may play a clearer role, particularly under inflammatory conditions characterized by a Th-2 response. Indeed, IL-13 treatment increased Cl− secretion in the airways of wild-type but not SLC26A9-deficient mice, with the latter thus exhibiting airway mucus obstruction [29]. SLC26A9-deficient mice also showed gastrointestinal manifestations, including decreased gastric acid secretion in a framework of extensive stomach pathology [37].
References
- Alper, S.L.; Sharma, A.K. The SLC26 Gene Family of Anion Transporters and Channels. Mol. Asp. Med. 2013, 34, 494–515.
- Geertsma, E.R.; Chang, Y.-N.; Shaik, F.R.; Neldner, Y.; Pardon, E.; Steyaert, J.; Dutzler, R. Structure of a Prokaryotic Fumarate Transporter Reveals the Architecture of the SLC26 Family. Nat. Struct. Mol. Biol. 2015, 22, 803–808.
- Mount, D.B.; Romero, M.F. The SLC26 Gene Family of Multifunctional Anion Exchangers. Pflug. Arch. Eur. J. Physiol. 2004, 447, 710–721.
- Wedenoja, S.; Pekansaari, E.; Höglund, P.; Mäkelä, S.; Holmberg, C.; Kere, J. Update on SLC26A3 Mutations in Congenital Chloride Diarrhea. Hum. Mutat. 2011, 32, 715–722.
- Everett, L.A.; Glaser, B.; Beck, J.C.; Idol, J.R.; Buchs, A.; Heyman, M.; Adawi, F.; Hazani, E.; Nassir, E.; Baxevanis, A.D.; et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 1997, 17, 411–422.
- Yoon, J.S.; Park, H.-J.; Yoo, S.-Y.; Namkung, W.; Jo, M.J.; Koo, S.K.; Park, H.-Y.; Lee, W.-S.; Kim, K.H.; Lee, M.G. Heterogeneity in the Processing Defect of SLC26A4 Mutants. J. Med. Genet. 2008, 45, 411–419.
- Dai, P.; Stewart, A.K.; Chebib, F.; Hsu, A.; Rozenfeld, J.; Huang, D.; Kang, D.; Lip, V.; Fang, H.; Shao, H.; et al. Distinct and Novel SLC26A4/Pendrin Mutations in Chinese and U.S. Patients with Nonsyndromic Hearing Loss. Physiol. Genom. 2009, 38, 281–290.
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211.
- Randell, S.H.; Boucher, R.C. Effective Mucus Clearance Is Essential for Respiratory Health. Am. J. Respir. Cell Mol. Biol. 2006, 35, 20–28.
- Button, B.; Cai, L.-H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. Periciliary Brush Promotes the Lung Health by Separating the Mucus Layer from Airway Epithelia. Science 2012, 337, 937–941.
- Birket, S.E.; Chu, K.K.; Liu, L.; Houser, G.H.; Diephuis, B.J.; Wilsterman, E.J.; Dierksen, G.; Mazur, M.; Shastry, S.; Li, Y.; et al. A Functional Anatomic Defect of the Cystic Fibrosis Airway. Am. J. Respir. Crit. Care Med. 2014, 190, 421–432.
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.V.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and Functional CFTR Channel Are Required for Proper Mucin Secretion and Link Cystic Fibrosis with Its Mucus Phenotype. J. Exp. Med. 2012, 209, 1263–1272.
- Pezzulo, A.A.; Tang, X.X.; Hoegger, M.J.; Abou Alaiwa, M.H.; Ramachandran, S.; Moninger, T.O.; Karp, P.H.; Wohlford-Lenane, C.L.; Haagsman, H.P.; van Eijk, M.; et al. Reduced Airway Surface PH Impairs Bacterial Killing in the Porcine Cystic Fibrosis Lung. Nature 2012, 487, 109–113.
- Nakayama, K.; Jia, Y.X.; Hirai, H.; Shinkawa, M.; Yamaya, M.; Sekizawa, K.; Sasaki, H. Acid Stimulation Reduces Bactericidal Activity of Surface Liquid in Cultured Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2002, 26, 105–113.
- Abou Alaiwa, M.H.; Beer, A.M.; Pezzulo, A.A.; Launspach, J.L.; Horan, R.A.; Stoltz, D.A.; Starner, T.D.; Welsh, M.J.; Zabner, J. Neonates with Cystic Fibrosis Have a Reduced Nasal Liquid pH.; A Small Pilot Study. J. Cyst. Fibros. 2014, 13, 373–377.
- Lohi, H.; Kujala, M.; Makela, S.; Lehtonen, E.; Kestila, M.; Saarialho-Kere, U.; Markovich, D.; Kere, J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J. Biol. Chem. 2002, 277, 14246–14254.
- Bertrand, C.A.; Zhang, R.; Pilewski, J.M.; Frizzell, R.A. SLC26A9 Is a Constitutively Active, CFTR-Regulated Anion Conductance in Human Bronchial Epithelia. J. Gen. Physiol. 2009, 133, 421.
- Liu, X.; Li, T.; Riederer, B.; Lenzen, H.; Ludolph, L.; Yeruva, S.; Tuo, B.; Soleimani, M.; Seidler, U. Loss of Slc26a9 Anion Transporter Alters Intestinal Electrolyte and HCO3− Transport and Reduces Survival in CFTR-Deficient Mice. Pflug. Arch. 2015, 467, 1261–1275.
- Kim, D.; Huang, J.; Billet, A.; Abu-Arish, A.; Goepp, J.; Matthes, E.; Tewfik, M.A.; Frenkiel, S.; Hanrahan, J.W. Pendrin Mediates Bicarbonate Secretion and Enhances Cystic Fibrosis Transmembrane Conductance Regulator Function in Airway Surface Epithelia. Am. J. Respir. Cell Mol. Biol. 2019, 60, 705–716.
- Kmit, A.; Marson, F.A.L.; Pereira, S.V.-N.; Vinagre, A.M.; Leite, G.S.; Servidoni, M.F.; Ribeiro, J.D.; Ribeiro, A.F.; Bertuzzo, C.S.; Amaral, M.D. Extent of Rescue of F508del-CFTR Function by VX-809 and VX-770 in Human Nasal Epithelial Cells Correlates with SNP Rs7512462 in SLC26A9 Gene in F508del/F508del Cystic Fibrosis Patients. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1323–1331.
- Sato, Y.; Thomas, D.Y.; Hanrahan, J.W. The Anion Transporter SLC26A9 Localizes to Tight Junctions and Is Degraded by the Proteasome When Co-Expressed with F508del–CFTR. J. Biol. Chem. 2019, 294, 18269–18284.
- Lam, A.-T.N.; Aksit, M.A.; Vecchio-Pagan, B.; Shelton, C.A.; Osorio, D.L.; Anzmann, A.F.; Goff, L.A.; Whitcomb, D.C.; Blackman, S.M.; Cutting, G.R. Increased Expression of Anion Transporter SLC26A9 Delays Diabetes Onset in Cystic Fibrosis. J. Clin. Investig. 2020, 130, 272–286.
- Larsen, M.B.; Choi, J.J.; Wang, X.; Myerburg, M.M.; Frizzell, R.A.; Bertrand, C.A. Separating the Contributions of SLC26A9 and CFTR to Anion Secretion in Primary Human.n Bronchial Epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L1147–L1160.
- Pinto, M.C.; Quaresma, M.C.; Silva, I.A.L.; Railean, V.; Ramalho, S.S.; Amaral, M.D. Synergy in Cystic Fibrosis Therapies: Targeting SLC26A9. Int. J. Mol. Sci. 2021, 22, 13064.
- Chi, X.; Jin, X.; Chen, Y.; Lu, X.; Tu, X.; Li, X.; Zhang, Y.; Lei, J.; Huang, J.; Huang, Z.; et al. Structural Insights into the Gating Mechanism of Human SLC26A9 Mediated by Its C-Terminal Sequence. Cell Discov. 2020, 6, 55.
- Single Cell Type—SLC26A9—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000174502-SLC26A9/single+cell+type (accessed on 27 December 2021).
- Walter, J.D.; Sawicka, M.; Dutzler, R. Cryo-EM Structures and Functional Characterization of Murine Slc26a9 Reveal Mechanism of Uncoupled Chloride Transport. eLife 2019, 8, e46986.
- Salomon, J.J.; Spahn, S.; Wang, X.; Füllekrug, J.; Bertrand, C.A.; Mall, M.A. Generation and Functional Characterization of Epithelial Cells with Stable Expression of SLC26A9 Cl− Channels. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L593–L602.
- Anagnostopoulou, P.; Riederer, B.; Duerr, J.; Michel, S.; Binia, A.; Agrawal, R.; Liu, X.; Kalitzki, K.; Xiao, F.; Chen, M.; et al. SLC26A9-Mediated Chloride Secretion Prevents Mucus Obstruction in Airway Inflammation. J. Clin. Investig. 2012, 122, 3629–3634.
- Xu, J.; Henriksnäs, J.; Barone, S.; Witte, D.; Shull, G.E.; Forte, J.G.; Holm, L.; Soleimani, M. SLC26A9 Is Expressed in Gastric Surface Epithelial Cells, Mediates Cl−/HCO3− Exchange, and Is Inhibited by NH4+. Am. J. Physiol.-Cell Physiol. 2005, 289, C493–C505.
- Chang, M.-H.; Plata, C.; Zandi-Nejad, K.; Sinđić, A.; Sussman, C.R.; Mercado, A.; Broumand, V.; Raghuram, V.; Mount, D.B.; Romero, M.F. Slc26A9—Anion Exchanger, Channel and Na+ Transporter. J. Membr. Biol. 2009, 228, 125–140.
- Amlal, H.; Xu, J.; Barone, S.; Zahedi, K.; Soleimani, M. The Chloride Channel/Transporter Slc26a9 Regulates the Systemic Arterial Pressure and Renal Chloride Excretion. J. Mol. Med. 2013, 91, 561–572.
- Chang, M.-H.; Plata, C.; Sindic, A.; Ranatunga, W.K.; Chen, A.-P.; Zandi-Nejad, K.; Chan, K.W.; Thompson, J.; Mount, D.B.; Romero, M.F. Slc26a9 Is Inhibited by the R-Region of the Cystic Fibrosis Transmembrane Conductance Regulator via the STAS Domain. J. Biol. Chem. 2009, 284, 28306–28318.
- Avella, M.; Loriol, C.; Boulukos, K.; Borgese, F.; Ehrenfeld, J. SLC26A9 Stimulates CFTR Expression and Function in Human Bronchial Cell Lines. J. Cell. Physiol. 2011, 226, 212–223.
- Ousingsawat, J.; Schreiber, R.; Kunzelmann, K. Differential Contribution of SLC26A9 to Cl− Conductance in Polarized and Non-Polarized Epithelial Cells. J. Cell. Physiol. 2012, 227, 2323–2329.
- Bertrand, C.A.; Mitra, S.; Mishra, S.K.; Wang, X.; Zhao, Y.; Pilewski, J.M.; Madden, D.R.; Frizzell, R.A. The CFTR Trafficking Mutation F508del Inhibits the Constitutive Activity of SLC26A9. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L912–L925.
- Xu, J.; Song, P.; Miller, M.L.; Borgese, F.; Barone, S.; Riederer, B.; Wang, Z.; Alper, S.L.; Forte, J.G.; Shull, G.E.; et al. Deletion of the Chloride Transporter Slc26a9 Causes Loss of Tubulovesicles in Parietal Cells and Impairs Acid Secretion in the Stomach. Proc. Natl. Acad. Sci. USA 2008, 105, 17955–17960.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
679
Revisions:
3 times
(View History)
Update Date:
18 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




