Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovambattista Pani | + 1674 word(s) | 1674 | 2022-01-24 09:44:01 | | | |
| 2 | Fiorella Sarubbo | Meta information modification | 1674 | 2022-01-26 14:47:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pani, G.; Sarubbo, F. Gut Microbiota Affect Neurogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/18746 (accessed on 07 February 2026).
Pani G, Sarubbo F. Gut Microbiota Affect Neurogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/18746. Accessed February 07, 2026.
Pani, Giovambattista, Fiorella Sarubbo. "Gut Microbiota Affect Neurogenesis" Encyclopedia, https://encyclopedia.pub/entry/18746 (accessed February 07, 2026).
Pani, G., & Sarubbo, F. (2022, January 25). Gut Microbiota Affect Neurogenesis. In Encyclopedia. https://encyclopedia.pub/entry/18746
Pani, Giovambattista and Fiorella Sarubbo. "Gut Microbiota Affect Neurogenesis." Encyclopedia. Web. 25 January, 2022.
Copy Citation
Adult neurogenesis (i.e., the life-long generation of new neurons from undifferentiated neuronal precursors in the adult brain) may contribute to brain repair after damage, and participates in plasticity-related processes including memory, cognition, mood and sensory functions. Among the many intrinsic (oxidative stress, inflammation, and ageing), and extrinsic (environmental pollution, lifestyle, and diet) factors deemed to impact neurogenesis, significant attention has been recently attracted by the myriad of saprophytic microorganismal communities inhabiting the intestinal ecosystem and collectively referred to as the gut microbiota.
gut microbiota
gut-brain axis
adult neurogenesis
physical activity
neurodegenerative disorders
ageing
1. Introduction
Neurogenesis can be defined as the generation of new neurons, glial cells and other neural lineages from neural stem cells (NSCs) and neural progenitor cells (NPCs) [1][2]. This process includes the maturation, migration and functional integration of NSCs or NPSs into the preexisting neuronal network [3][4]. When it occurs in adult life, it is known as adult neurogenesis (AN). Although NSCs are present in several brain regions, the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricle are the main AN niches [5]. AN in other adult brain regions (e.g., the neocortex, striatum, amygdala and substantia nigra) is limited under normal physiological conditions, but could be induced after injury [6]. Maintenance of neurogenesis contributes to brain repair after damage and is believed to play a role in stress-responses and higher functions involving brain plasticity such as memory and cognition [7][8][9][10][11], mood [12], or perceptual (e.g., olfactory) learning [13][14]. Accordingly, an impairment in neurogenesis, as seen during ageing or in pathological conditions [15], has been associated with seizures [16][17], depression [18], and decline of learning abilities [19]. Impaired neurogenesis may occur because of a reduction in the number and/or function of NSCs and NPCs [20]. This may be due to the synergic action of several mechanisms operating in the brain in ageing or neurodegenerative conditions: inflammation [21][22], oxidative stress [23], or toxic substances like short-chain fatty acids (SCFAs), branched chain amino acids and peptidoglycans, originating from an altered intestinal microbiota [24]. Gut-resident microbial communities are in turn modulated by extrinsic factors, such as lifestyle and diet; importantly, imbalances affecting this complex ecosystem can impact the permeability of the body barriers, including the blood brain barrier (BBB) and the enteric barrier, so as to allow the passage of potentially noxious substances to brain tissue along the so-called gut-brain axis (GBA) [25][26].
To counteract the deterioration of neurogenesis, mechanisms that could be exogenously regulated, such as the composition of gut microbiota, are of particular interest. Gut microbiota is comprised of several species of microorganisms, including bacteria, yeast, and viruses [27], cohabiting in a delicate balance whose disruption (dysbiosis) can lead to aberrant neural and glial reactivity accompanied by loss of neurogenic ability [28]. Thus, a functional relationship links microbiota, GBA and neurogenesis [29][25], and alterations in this axis not only affect the neural regulation of the gastrointestinal tract, but, also contribute to several brain disturbances, such as mood (e.g., depression, anxiety) and neurodevelopmental (e.g., autism) [30][31] and cognitive disorders (e.g., Alzheimer’s disease) [25][32][33][34]. Therefore, in establishing a bidirectional connection between enteric microbes and the brain, GBA exploits several anatomic structures, systems, and metabolic routes [25], such as the neuroendocrine (by the hypothalamic–pituitary–adrenal (HPA) axis) and neuroimmune systems, the sympathetic and parasympathetic arms of the autonomic nervous system, including the enteric nervous system, the vagus nerve [35], and the immune system. Not surprisingly, therefore, the GBA has been portrayed as a “second brain” [25].
As an additional layer of complexity, many factors can influence microbiota composition, including infection, mode of birth delivery, use of antibiotic medications, the nature of nutritional provision, environmental stressors, host genetics and ageing [36][37]. Among the potential therapeutic approaches aimed at the microbiota to target GBA and neurogenesis, diet composition appears particularly attractive for its feasibility. For instance, natural antioxidants and anti-inflammatory molecules, such as dietary polyphenols, have long been investigated as potential adjuvants to support AN [38]. In simple terms, maintaining a healthy brain across the lifespan [39] may simply require “good” intestinal bacteria and the right diet to keep them going.
2. Connection between Intestinal Microbiota and Neurogenesis
Several clinical and experimental studies point to a functional connection between intestinal microbiota and neurogenesis through the GBA. This emerging evidence implies that microbiota composition may represent both a causative determinant and a therapeutic target in diseases where neurogenesis plays a key role [39][40][41][42][43]. Experimental data in support of the influence of microbiota on AN can be grouped in four general domains: (a) data from Germ-free (GF) animals; (b) data on substances derived from bacterial fermentation of food; (c) changes in bacteria homeostasis due to exogenous factors (e.g., antibiotics or stress); (d) consequences of dietary changes (Figure 1):
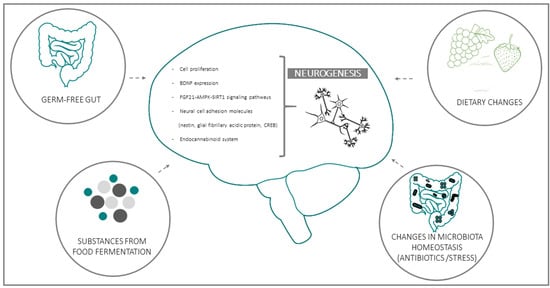
Figure 1. Schematic representation of the four main experimental models used to investigate the functional linkages between intestinal bacteria and adult (mainly hippocampal) neurogenesis. Biochemical and functional parameters employed in most studies for the evaluation of neurogenesis and its microbiota-induced modifications are listed in the central, brain-shaped field.
(a) GF gut: GF animal models, usually mice or rats, grown up without any exposure to microorganisms, constitute an essential tool in studying the influence of the gut microbiota on brain function; not surprisingly, one of the first studies that highlighted the effect of the microbiota on neurogenesis was conducted on this model.
Using bromo-2-deoxyuridine (BrdU) immunohistochemistry, it was shown that, compared to conventionally raised mice, GF and GF–colonized mice exhibited a trend to increased cell proliferation, predominantly in the dorsal hippocampus, accompanied by alterations in the hippocampal brain-derived neurotrophic factor (BDNF) [10]. In agreement, another study reported an altered expression of synaptic plasticity-related genes, with significantly lower BDNF mRNA expression in the hippocampus, amygdala, and cingulate cortex in GF mice; of note, these areas participate in neurogenesis and are key components of the neural circuitry underlying behaviour. Along similar lines, Kundu and colleagues [40], investigated the effects of transplanting the gut microbiota from young or old donor mice into young GF recipient mice [40]. They found that the transplant-induced hippocampal AN is in parallel with the activation of the pro-neurogenic FGF21-AMPK-SIRT1 signalling pathway. Moreover, it has been observed that intestinal bacteria and components of the bacterial cell wall maintain the adult enteric neuron system and nitrergic neurons by promoting intestinal neurogenesis via the Toll-like Receptor 2 (TLR2) [44].
(b) Substances produced by food fermentation: Converging lines of evidence point to the potential role of food fermentation substances produced by gut bacteria on the modulation of AN. This is the case of the SCFA butyrate, synthesized from non-absorbed carbohydrates by colonic microbiota [45]. In an animal model of ischemia it was demonstrated that the histone deacetylase inhibitor, sodium butyrate, stimulates the incorporation of BrdU in the subgranular and the subventricular zone of the hippocampus, striatum, and frontal cortex in rats subjected to permanent cerebral ischemia. This treatment also increased the number of cells expressing the polysialic acid-neural cell adhesion molecule, nestin, the glial fibrillary acidic protein, the phospho-cAMP response element-binding protein (CREB), and BDNF in various brain regions after brain ischemia [46]. Accordingly, it was also demonstrated that oral sodium butyrate impacts brain metabolism and hippocampal neurogenesis in pigs [47].
(c) Changes in bacteria homeostasis due to exogenous factors (e.g., antibiotics or stress): Prompted by the emerging notion that the intestinal ecosystem can influence the vegetative and cognitive functions of the host [48][49], several studies have focused on the impact of antibiotics on microbiota and gut-brain communication. In mice, depleting gut microbiota with antibiotics, from weaning onward, induces cognitive deficits, specifically in memory retention, and leads to a significant reduction of BDNF in the adult brain, maybe by the involvement in AN [50]. However, while consistent with the observed changes, a specific impact of microbiota depletion on neurogenesis was not directly demonstrated in this study. This aspect was instead specifically addressed by Môhle et al. [51], who reported a long-lasting impairment in neurogenesis, accompanied by behaviour deficits in antibiotic-treated mice. It is worthy of note that these alterations were partially restored by exercise (running) and probiotics administration. Mechanistically, the above treatments increased the number of Ly6C(hi) monocytes [51], a cell population involved in immune surveillance and host defense upon infections and inflammation. Moreover, elimination of Ly6Chi monocytes by antibody depletion or by using knockout mice resulted in decreased neurogenesis, whereas the adoptive transfer of Ly6Chi monocytes was able to preserve neurogenesis after antibiotic treatment [51].
Besides antibiotics, the homeostasis of intestinal microbiota can also be affected by other drugs and stress factors. Chronic stress can impact gut microbiota diversity, promoting an increase in pathogenic bacteria at the expense of beneficial ones (dysbiosis). This imbalance, in turn, affects lipid metabolism and decreases the endocannabinoid signalling system, thus reducing hippocampal AN. Of note, dysbiosis frequently accompanies ageing and may lead to chronic inflammation and a decrease in pro-neurogenic bacterial metabolites (such as SCFAs) in the senescent intestine [52].
(d) Dietary changes: High-fat or choline-deficient diets produce a specific gut microbiota signature in the small intestine and cecum, marked by increased propionate and butyrate synthesis, mitochondrial biogenesis and generation of reactive oxidative species (ROS) downstream of SCFAs. All of these variations affect NSCs fate, leading to premature differentiation and depletion of the NSC pool in the AN niches of high-fat or choline-deficient-fed mice, ultimately impairing AN [39]. On the other hand, dietary or probiotic interventions have been indicated as effective therapeutic approaches to fight stress-associated neurological disturbances operating through the GBA [8]. Importantly, a clinical study on bacterial strains known to boost neurogenesis in mice reported improved cognitive functions in adult patients with major depression; while the involvement of neurogenesis in the effects observed in human subjects can be only indirectly inferred; the consistency with results gleaned in the preclinical setting is intriguing [53][54]. Furthermore, with regard to probiotics, it was found that in a rat model of early-life stress, maternal separation caused a marked decrease in hippocampal BDNF, while the probiotic Bifidobacterium breve 6330 increased BDNF to levels observed in control animals, suggesting that BDNF might be involved in the regulation of anxiety through microbiome-GBA [55]. Thus, diet and probiotics represent major environmental determinants of the gut flora composition [56] and, as such, constitute potential tools for the restoration and maintenance of brain homeostasis.
References
- Gage, F.H.F. Mammalian neural stem cells. Science 2000, 287, 1433–1438.
- Zhao, C.; Deng, W.; Gage, F. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660.
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686.
- Riquelme, P.A.; Drapeau, E.; Doetsch, F. Brain micro-ecologies: Neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 123–137.
- Cavallucci, V.; Fidaleo, M.; Pani, G. Neural Stem Cells and Nutrients: Poised Between Quiescence and Exhaustion. Trends. Endocrinol. Metab. 2016, 27, 756–769.
- Mirescu, C.; Gould, E. Stress and adult neurogenesis. Hippocampus 2006, 16, 233–238.
- Apple, D.M.; Solano-Fonseca, R.; Kokovay, E. Neurogenesis in the aging brain. Biochem. Pharmacol. 2017, 141, 77–85.
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363.
- Colangelo, A.; Cirillo, G.; Alberghina, L.; Papa, M.; Westerhoff, H. Neural plasticity and adult neurogenesis: The deep biology perspective. Neural Regen. Res. 2019, 14, 201–205.
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, e7–e9.
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350.
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346.
- Lazarini, F.; Lledo, P.M. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011, 34, 20–30.
- Braun, S.M.G.; Jessberger, S. Adult neurogenesis: Mechanisms and functional significance. Development 2014, 141, 1983–1986.
- Mattson, M.P. Neuroprotective signaling and the aging brain: Take away my food and let me run. Brain Res. 2000, 886, 47–53.
- Jessberger, S.; Nakashima, K.; Clemenson, G.D.; Mejia, E.; Mathews, E.; Ure, K.; Ogawa, S.; Sinton, C.M.; Gage, F.H.; Hsieh, J. Epigenetic Modulation of Seizure-Induced Neurogenesis and Cognitive Decline. J. Neurosci. 2007, 27, 5967–5975.
- Kron, M.M.; Zhang, H.; Parent, J.M. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 2010, 30, 2051–2059.
- Dokter, M.; von Bohlen und Halbach, O. Neurogenesis within the adult hippocampus under physiological conditions and in depression. Neural Regen. Res. 2012, 7, 552–559.
- Leuner, B.; Gould, E.; Shors, T.J. Is there a link between adult neurogenesis and learning? Hippocampus 2006, 16, 216–224.
- Walgrave, H.; Balusu, S.; Snoeck, S.; Vanden Eynden, E.; Craessaerts, K.; Thrupp, N.; Wolfs, L.; Horré, K.; Fourne, Y.; Ronisz, A.; et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease. Cell Stem Cell 2021, 28, 1805–1821.
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254.
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637.
- Taupin, P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 16–21.
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Liu, C.; Yang, S.Y.; Wang, L.; Zhou, F. The gut microbiome: Implications for neurogenesis and neurological diseases. Neural Regen. Res. 2022, 17, 53–58.
- Hanslik, K.; Marino, K.; Ulland, T. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front Cell Neurosci. 2021, 15, 718324.
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of regressive autism. Anaerobe 2012, 18, 260–262.
- Patusco, R.; Ziegler, J. Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: AN update for practitioners. Adv. Nutr. 2018, 9, 637–650.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14.
- Köhler, C.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.; Carvalho, A. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166.
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643.
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528.
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184.
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605.
- Sarubbo, F.; Moranta, D.; Pani, G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018, 90, 456–470.
- Ribeiro, M.F.; Santos, A.A.; Afonso, M.B.; Rodrigues, P.M.; Sá Santos, S.; Castro, R.E.; Rodrigues, C.M.P.; Solá, S. Diet-dependent gut microbiota impacts on adult neurogenesis through mitochondrial stress modulation. Brain Commun. 2020, 2, fcaa165.
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.X.Y.; Kim, H.; Faylon, L.E.; Martin, K.A.; Purbojati, R.; Drautz-Moses, D.I.; Ghosh, S.; et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760.
- Pearson-Leary, J.; Zhao, C.; Bittinger, K.; Eacret, D.; Luz, S.; Vigderman, A.S.; Dayanim, G.; Bhatnagar, S. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol. Psychiatry 2020, 25, 1068–1079.
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245.
- Cerdó, T.; Diéguez, E.; Campoy, C. Impact of gut microbiota on neurogenesis and neurological diseases during infancy. Curr. Opin. Pharmacol. 2020, 50, 33–37.
- Yarandi, S.S.; Kulkarni, S.; Saha, M.; Sylvia, K.E.; Sears, C.L.; Pasricha, P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology 2020, 159, 200–213.
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528.
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240.
- Val-Laillet, D.; Guérin, S.; Coquery, N.; Nogret, I.; Formal, M.; Romé, V.; Le Normand, L.; Meurice, P.; Randuineau, G.; Guilloteau, P.; et al. Oral sodium butyrate impacts brain metabolism and hippocampal neurogenesis, with limited effects on gut anatomy and function in pigs. FASEB J. 2018, 32, 2160–2171.
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Mayer, E.A. Gut feelings: The emerging biology of gut-”brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466.
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173.
- Möhle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6Chi Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956.
- Romo-Araiza, A.; Ibarra, A. Prebiotics and probiotics as potential therapy for cognitive impairment. Med. Hypotheses 2020, 134, 109410.
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222.
- Ishikawa, R.; Fukushima, H.; Nakakita, Y.; Kado, H.; Kida, S. Dietary heat-killed Lactobacillus brevis SBC8803 (SBL88TM) improves hippocampus-dependent memory performance and adult hippocampal neurogenesis. Neuropsychopharmacol. Rep. 2019, 39, 140–145.
- O’Sullivan, E.; Barrett, E.; Grenham, S.; Fitzgerald, P.; Stanton, C.; Ross, R.P.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes 2011, 2, 199–207.
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
870
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
27 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




