
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alok Paul | + 3300 word(s) | 3300 | 2022-01-20 07:58:01 | | | |
| 2 | Lindsay Dong | Meta information modification | 3300 | 2022-01-25 03:32:51 | | |
Video Upload Options
Phytopharmaceuticals have been widely used globally since ancient times and acknowledged by healthcare professionals and patients for their superior therapeutic value and fewer side effects compared to modern medicines. Dose reduction, improved bioavailability, receptor-selective binding, and targeted delivery of phytopharmaceuticals can be likely achieved by molding them into specific nano-formulations.
1. Introduction
Nanotechnology is a vital tool for medical sciences. The introduction of nanomedicine, using nanotechnology combined with drugs or diagnostic molecules, has improved the ability to target specific cells or tissues that require treatment and repair. These nanomaterials have been proven to be produced at a nanoscale level and are safe to introduce into the body. It is possible to modify a variety of nanocarriers’ characteristics that include their constituents, size, shape, bioavailability, surface properties, and target specificity to achieve or enhance desirable pharmacological targets [1][2] Several strategies have been implemented to increase the drug-target specificity. Recently, several studies have reported improved efficacy of therapy when combined with nanomaterials [3]. Pure herbal medicines are often considered less effective compared to pure constituents that are mainly demonstrated to have reduced intestinal absorption when administered orally [4]. This is the reason behind the pharmacological activity/loss associated with pure constituents and such problems can be overcome using new drug delivery systems, such as nanotechnology.
Nanoparticulate delivery of drugs can generally improve drugs’ solubility, bioavailability, stability, pharmacological activity, increase target specificity, promote transport across membrane, prolong circulation times, and reduce systemic and organ toxicity [5][6]. Various treatments are being investigated with the use of nanoparticle drug delivery systems for diseases, such as infectious diseases, autoimmune diseases, cardiovascular diseases, neurodegenerative diseases, ocular diseases, fungal infections, iron deficiency, and pulmonary diseases [7][8]. However, the greatest advances were seen in the treatment of cancer with several nano strategies being used clinically after approval by the FDA in the United States of America [9]. Recently, more attention has shifted towards novel drug delivery systems using nanoparticles for herbal and plant-based drugs [10].
Plant-based medicine has some limitations which hinder its use and production in the mainstream disease treatment and therapy. There are several chemical constituents in a plant’s extract that lead to its medicinal properties. The active constituents of plant extracts like tannins, flavonoids, alkaloids, phenylpropanoids, and terpenoids are water-soluble but show poor absorption from their inability to cross lipid membranes and have large molecular sizes, which then results in low bioavailability and efficacy [5][11][12]. There are also concerns of safety due to the incompatibility of some plant extracts with other components in a drug formulation which can lead to undesirable effects [11]. High systemic clearance of these compounds also leads to low therapeutic levels in the blood resulting in no therapeutic effect [13]. Furthermore, poor reproducibility of in vitro effects in vivo prevents many plant-based medicines from clearing clinical trial phases [14]. Nanomedicine aims to overcome these limitations and to improve the delivery of plant-based medicines to treat various diseases. Nanoparticle drug delivery systems can potentially improve the stability, solubility, and bioavailability of encapsulated plant extracts, promote its movement across lipid membranes, and prolong its circulation, all while delivering the active constituent to a specific target site [5][6][11] (Figure 1). Liposomes, dendrimers, polymeric NCs, polymeric micelles, metallic NPs (magnetic, gold), SLNs, nanocapsules, nanospheres, and nanogels are some of the examples of nano-based drug delivery systems that are presently under investigation [10].
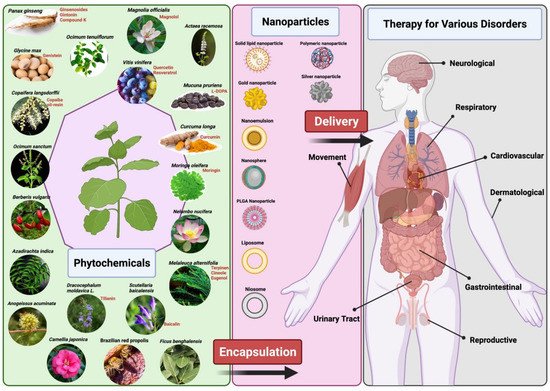
2. Therapeutic Applications of Nano-Phytopharmaceuticals
2.1. Nano-Phytopharmaceuticals in Neurological (CNS) Disorders
Despite enduring efforts to deliver medicinal drugs to the brain tissue, these treatment regimens are compromised by low bioavailability due to the blood-brain barrier (BBB), a natural protective layer consisting of capillary endothelial cells, pericytes, and tight junctions [15]. This near-impermeable barrier impedes the entry of most macromolecules and allows only the minutest particles (<400 Da) to cross into the nerve tissue. Indeed, less than 5% of conventional therapeutic molecules in various stages of pharmaceutical development may penetrate this physiological barrier [16]. Therefore, CNS-targeted natural product formulations in nanocarriers hold infinite promise. Evidence suggests that nutraceuticals and phytochemicals exert therapeutic effects in neurological diseases, owing to their antioxidant, anti-inflammatory, and neuroprotective mechanisms [17][18]. Coupled with nanoscale delivery systems which improve solubility, enhance retention rates [19], and with the ability to permeate through the BBB, there is hope yet for effective treatment of neurological disorders. The following topic discusses two herbal compounds, curcumin and ginseng, and their significant potential for use in neurotherapeutics through nano-encapsulation.
2.2. Nano-Phytopharmaceuticals in Cardiovascular Disorders
Since CVD require most often long-term medication, treatment regimens become complex and create a burden for the patient especially when multiple medicines are prescribed and must be taken for life [32][33] Although these therapeutic drugs have been successful in halting the progression of the disease, thereby improving the quality of life of patients, most of these cures only the symptoms and may not repair or regenerate the damaged tissues. In addition, CVD medication has different side effects, such as antiplatelet drugs may cause diarrhea, rash, or itching [32]. Others can cause abdominal pain, headache, chest pain, muscle aches, and dizziness. In the case of anticoagulants, their side effects can lead to bleeding and necrotic or gangrenous skin. Given the excessive side effects of current therapies, alternative therapeutic approaches like medicinal plants and natural products are preferred. Against this premise, a better treatment for CVD that would not burden the patients is necessary. Hence, it is important to explore new technologies and drugs to lessen the use of conventional treatments. The lower toxicity, chemical diversity, cost-effectiveness, and therapeutic potentials of natural products make them the popular choice of medicine compared to other products [34]. With the combination of nanoformulation methods to deliver phytomedicines, it becomes more effective with improved solubility, bioavailability, circulation time, surface area-to-volume ratio, nil systemic adverse side effects, and drug delivery efficiency of these medications. The introduction of nanomedicine using a combined nanotechnology with drugs or diagnostic molecules has improved the ability to target specific cells or tissues that require treatment and repair. These nanomaterials are produced on a nanoscale level and are safe to introduce into the body. Hence, applications for nanotechnology in medicine include imaging, diagnosis, or the delivery of drugs that will help medical professionals treat various diseases including cardiovascular diseases [35][36]. The functionality of the most recent nano-formulated medicinal plants and/or natural products against various cardiovascular conditions such as hypertension, atherosclerosis, thrombosis, and myocardial infarction is expected to be maximized.
Some plant extracts/compounds used for treatment of CVD that are nano-formulated for efficient delivery to their target are listed in Table 1. Among the extracts used for CVD, curcumin, quercetin, and resveratrol were the most applied natural products, respectively. However, curcumin, despite its curative potential, has poor aqueous solubility and consequently, minimal systemic bioavailability along with rapid degradation [37]. These characteristics restrict the utilization of curcumin a medical perspective. Liposomes have found uses in drug delivery of poorly water-soluble drugs. Such drug-loaded liposomes can be fabricated by a wide variety of nanotechnology methods such as ethanol injection, thin-film hydration, sonication, high-pressure extrusion, reverse-phase evaporation, calcium-induced fusion, and supercritical fluid methods, among others [37].
| Nanoformulation | Phyto-Pharmaceutical | Effects | References |
|---|---|---|---|
| Liposomes | Curcumin | Anti-hypercholesterolemic, anti-atherosclerotic and protective against cardiac ischemia and reperfusion. | [38] |
| PLGA nanoparticle | Quercetin | Anti-hypercholesterolemia, better cell rescue by lowering oxidized thiols and sustaining superior ATP production, improved therapeutics for ROS-based cardiac diseases. | [39] |
| Solid lipid Nanoparticle | Resveratrol | Protective action of vascular walls towards oxidation, inflammation, platelet oxidation and thrombus formation | [40][41] |
| 1,2-diacyl-Sn-glycero-3-phosphocholine [EPC] and 1,2-dipalmitoyl-Sn-glycero-3-phosphocholine (DPPC) liposomes | Magnolol | Enhanced inhibitory effect on migration and hyperplasia of vascular smooth-muscle cells; Anti-platelet, anti-thrombotic, and anti-hypertensive via inhibiting MAPK family activation, Akt/ERK1/2/GSK3 β-catenin pathway, and angiotensin-converting enzyme (ACE)/angiotensin II (Ang II)/Ang II type 1 receptor (AT-1R) cascade and upregulating PPAR-β/γ and NO/guanosine 3′,5′-cyclic phosphate/PKG. | [34][42][43] |
| Nano-micelles | Tilianin | Protective effects of cardiomyocytes by inhibiting inflammation and oxidative stress during myocardial ischemia-reperfusion injury | [44] |
| PEGylated nanostructured lipid carriers | Baicalin | Improved myocardial ischemia; beneficial roles against the initiation and progression of CVDs such as atherosclerosis, hypertension, myocardial infarction, reperfusion and heart failure | [45][46] |
| Liposomes | Berberine | Effect of protecting heart failure, hypertension, hyperlipidemia, insulin resistance, arrhythmias, and platelet aggregation. | [47][48] |
2.3. Nano-Phytopharmaceuticals in Pulmonary Disorders
2.4. Nano-Phytopharmaceuticals in Gastro-Intestinal Disorders
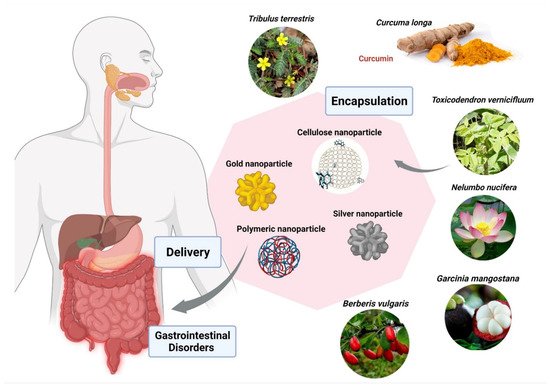
| Nanoformulation | Phyto-Pharmaceutical | Enteropathogen/GI Cell Lines; IC50/MIC | Reference |
|---|---|---|---|
| Polymeric (EPO) | Berberis vulgaris | Entamoeba histolytica; 26 ppm | [81] |
| Polymeric (EPO) | Curcuma longa | Entamoeba histolytica; 19 ppm | [81] |
| Polymeric (Cerium oxide) | Nelumbo nucifera | Human colon cancer (HCT 116); 4.16 µg/mL | [81] |
| Cellulose (Ethyl) | Garcinia mangostana | Helicobacter pylori; 62.5 µg/mL | [81] |
| Metallic (Silver) | Toxicodendron vernicifluum | Helicobacter pylori; 18.14 µg/mL | [81] |
| Metallic (Silver) | Toxicodendron vernicifluum | E.coli; 8.12 µg/mL | [81] |
| Metallic (Gold) | Tribulus terrestris | Helicobacter pylori; 16.75 µg/mL | [81] |
References
- Mobasser, S.; Firoozi, A.A. Review of nanotechnology applications in science and engineering. J. Civil. Eng. Urban. 2017, 64, 84–93.
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885.
- Morelli, L.; Gimondi, S.; Sevieri, M.; Salvioni, L.; Guizzetti, M.; Colzani, B.; Palugan, L.; Foppoli, A.; Talamini, L.; Morosi, L.; et al. Monitoring the fate of orally administered PLGA nanoformulation for local delivery of therapeutic drugs. Pharmaceutics 2019, 11, 658.
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128.
- Kumari, P.; Luqman, S.; Meena, A. Application of the combinatorial approaches of medicinal and aromatic plants with nanotechnology and its impacts on healthcare. DARU J. Pharm. Sci. 2019, 27, 475–489.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Mishra, V.; Kesharwani, P.; Amin, M.C.M.; Iyer, A. (Eds.) Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes, 1st ed.; 2021; Available online: https://www.elsevier.com/books/nanotechnology-based-approaches-for-targeting-and-delivery-of-drugs-and-genes/mishra/978-0-12-809717-5 (accessed on 20 August 2021).
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193.
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381.
- Prairna; Paramanya, A.; Sharma, S.; Bagdat, R.B.; Ali, A. Recent practices of medicinal and aromatic plants in nanotechnology. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 435–467.
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1–15.
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559.
- Namdari, M.; Eatemadi, A.; Soleimaninejad, M.; Hammed, A.T. A brief review on the application of nanoparticle enclosed herbal medicine for the treatment of infective endocarditis. Biomed. Pharmacother. 2017, 87, 321–331.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Guerra, M.; Blázquez, J.L.; Rodríguez, E.M. Blood–brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS 2017, 14, 1–15.
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493.
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in neurological disorders. Int. J. Mol. Sci. 2020, 21, 4424.
- Uddin, S.; Hossain, F.; Al Mamun, A.; Shah, M.A.; Hasana, S.; Bulbul, I.J.; Sarwar, S.; Mansouri, R.A.; Ashraf, G.M.; Rauf, A.; et al. Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Sci. Total Environ. 2020, 725, 138313.
- Zhu, F.-D.; Hu, Y.-J.; Yu, L.; Zhou, X.-G.; Wu, J.-M.; Tang, Y.; Qin, D.-L.; Fan, Q.-Z.; Wu, A.-G. Nanoparticles: A hope for the treatment of inflammation in CNS. Front. Pharmacol. 2021, 12, 683935.
- Schmitt, C.; Lechanteur, A.; Cossais, F.; Bellefroid, C.; Arnold, P.; Lucius, R.; Held-Feindt, J.; Piel, G.; Hattermann, K. Liposomal encapsulated curcumin effectively attenuates neuroinflammatory and reactive astrogliosis reactions in glia cvells and organotypic brain slices. Int. J. Nanomed. 2020, ume 15, 3649–3667.
- Alphandéry, E. Nano-therapies for glioblastoma treatment. Cancers 2020, 12, 242.
- Ege, D. Action mechanisms of curcumin in Alzheimer’s disease and its brain targeted delivery. Materials 2021, 14, 3332.
- Sadegh Malvajerd, S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 2019, 10, 728–739.
- Jeon, S.G.; Cha, M.-Y.; Kim, J.-I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.-J.; Moon, M. Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 297–307.
- Kim, J.W.; Cho, C.H.; Hwang, Y.-G.; Park, W.J.; Kang, H.; Kim, D.-O. Protective effects of red ginseng treated with gold nanoparticles against H2O2 -induced oxidative stress in neuronal PC-12 cells. Korean J. Food Sci. Technol. 2017, 49, 222–227.
- Yang, L.; Li, C.-L.; Tsai, T.-H. Preclinical herb-drug pharmacokinetic interaction of Panax ginseng extract and selegiline in freely moving rats. ACS Omega 2020, 5, 4682–4688.
- Pattan, G.; Kaul, G. Health hazards associated with nanomaterials. Toxicol. Ind. Heal. 2012, 30, 499–519.
- Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.-F.; Li, B.; Ge, C.; Zhou, G. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Lett. 2008, 183, 72–80.
- Pisanic, T.R.; Blackwell, J.D.; Shubayev, V.I.; Fiñones, R.R.; Jin, S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581.
- Zhao, J.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. NeuroToxicology 2009, 30, 220–230.
- Cha, K.E.; Myung, H. Cytotoxic effects of nanoparticles assessed in vitro and in vivo. J. Microbiol. Biotechnol. 2007, 17, 1573–1578.
- Van Der Laan, D.M.; Elders, P.J.M.; Boons, C.C.L.M.; Nijpels, G.; Krska, J.; Hugtenburg, J.G. The impact of cardiovascular medication use on patients’ daily lives: A cross-sectional study. Int. J. Clin. Pharm. 2018, 40, 412–420.
- Chronic kidney disease in the United States. 2021. Available online: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (accessed on 2 June 2021).
- Hesari, M.; Mohammadi, P.; Khademi, F.; Shackebaei, D.; Momtaz, S.; Moasefi, N.; Farzaei, M.H.; Abdollahi, M. Current Advances in the use of nanophytomedicine therapies for human cardiovascular diseases. Int. J. Nanomed. 2021, 16, 3293–3315.
- Owen, A.; Dufès, C.; Moscatelli, D.; Mayes, E.; Lovell, J.F.; Katti, K.V.; Sokolov, K.; Mazza, M.; Fontaine, O.; Rannard, S.; et al. The application of nanotechnology in medicine: Treatment and diagnostics. Nanomedicine 2014, 9, 1291–1294.
- Wang, D.K.; Rahimi, M.; Filgueira, C.S. Nanotechnology applications for cardiovascular disease treatment: Current and future perspectives. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102387.
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698.
- Salehi, B.; Prado-Audelo, D.; María, L.; Cortés, H.; Leyva-Gómez, G.; Stojanović-Radić, Z.; Singh, Y.D.; Patra, J.K.; Das, G.; Martins, N.; et al. Therapeutic applications of curcumin nanomedicine formulations in cardiovascular diseases. J. Clin. Med. 2020, 9, 746.
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramirez, J.; Figueiredo, H.; García-Rivas, G. Nanoencapsulated quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxidative Med. Cell. Longev. 2019, 2019, 1–14.
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250.
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791.
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, toxicity, bioavailability, and formulation of magnolol: An update. Front. Pharmacol. 2021, 12, 632767.
- Chen, C.Y.-C.; Wu, C.-H. Magnolol encapsulated by liposome in inhibiting smooth muscle cell proliferation. J. Chin. Chem. Soc. 2008, 55, 517–521.
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109.
- Zhang, S.; Wang, J.; Pan, J. Baicalin-loaded PEGylated lipid nanoparticles: Characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016, 23, 3696–3703.
- Xin, L.; Gao, J.; Lin, H.; Qu, Y.; Shang, C.; Wang, Y.; Lu, Y.; Cui, X. Regulatory mechanisms of baicalin in cardiovascular diseases: A review. Front. Pharmacol. 2020, 11, 583200.
- Xia, L.-M.; Luo, M.-H. Study progress of berberine for treating cardiovascular disease. Chronic Dis. Transl. Med. 2015, 1, 231–235.
- Duong, T.; Isomäki, A.; Paaver, U.; Laidmäe, I.; Tõnisoo, A.; Yen, T.; Kogermann, K.; Raal, A.; Heinämäki, J.; Pham, T.-M.-H. Nanoformulation and evaluation of oral berberine-loaded liposomes. Molecules 2021, 26, 2591.
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290.
- Shahraki, A.; Bahadorikhalili, S.; Hashemzaei, M.; Hajinezhad, M.; Afsharimoghaddam, A.; Sarani, F.; Tajrobekar, O. Resveratrol nano-capsule as an efficient tool for blood pressure regulation: A study on metabolic syndrome induced mice. Biosci Biotechnol. Res Commun. 2017, 10, 623–630.
- Shen, P.; Zhang, Z.; He, Y.; Gu, C.; Zhu, K.; Li, S.; Li, Y.; Lu, X.; Liu, J.; Zhang, N.; et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76.
- Stefanache, A.; Ignat, M.; Peptu, C.A.; Diaconu, A.; Stoleriu, I.; Ochiuz, L. Development of a prolonged-release drug delivery system with magnolol loaded in amino-functionalized mesoporous silica. Appl. Sci. 2017, 7, 237.
- Sheng, Y.-L.; Xu, J.-H.; Shi, C.-H.; Li, W.; Xu, H.-Y.; Li, N.; Zhao, Y.-Q.; Zhang, X.-R. UPLC-MS/MS-ESI assay for simultaneous determination of magnolol and honokiol in rat plasma: Application to pharmacokinetic study after administration emulsion of the isomer. J. Ethnopharmacol. 2014, 155, 1568–1574.
- Wang, Y.-J.; Chien, Y.-C.; Wu, C.-H.; Liu, D.-M. Magnolol-loaded core–shell hydrogel nanoparticles: Drug release, intracellular uptake, and controlled cytotoxicity for the inhibition of migration of vascular Ssooth muscle cells. Mol. Pharm. 2011, 8, 2339–2349.
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756.
- Natan, M.; Banin, E. From Nano to Micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322.
- Hayat, S.; Muzammil, S.; Fakhar-E-Alam, M.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A.; et al. Nanoantibiotics future nanotechnologies to combat antibiotic resistance. Front. Biosci. 2018, 10, 352–374.
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65.
- Borase, H.P.; Salunke, B.K.; Salunkhe, R.B.; Patil, C.D.; Hallsworth, J.E.; Kim, B.S.; Patil, S.V. Plant Extract: A Promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 173, 1–29.
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A.; et al. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 1845–1865.
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650.
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356.
- Lee, S.Y.; Krishnamurthy, S.; Cho, C.-W.; Yun, Y.-S. Biosynthesis of gold nanoparticles Using Ocimum sanctum extracts by solvents with different polarity. ACS Sustain. Chem. Eng. 2016, 4, 2651–2659.
- Lü, S.; Wu, Y.; Liu, H. Silver nanoparticles synthesized using Eucommia ulmoides bark and their antibacterial efficacy. Mater. Lett. 2017, 196, 217–220.
- Aiad, I.; Marzouk, M.; Shaker, S.A.; Ebrahim, N.E.; Abd-Elaal, A.; Tawfik, S.M. Antipyrine cationic surfactants capping silver nanoparticles as potent antimicrobial agents against pathogenic bacteria and fungi. J. Mol. Liq. 2017, 243, 572–583.
- Kuppusamy, P.; Ichwan, S.J.A.; Parine, N.R.; Yusoff, M.; Maniam, G.P.; Govindan, N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 2015, 29, 151–157.
- Rosa, R.M.; Silva, J.C.; Sanches, I.S.; Henriques, C. Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett. 2017, 207, 145–148.
- Huy, T.Q.; Thanh, N.T.H.; Thuy, N.T.; Van Chung, P.; Hung, P.N.; Le, A.-T. Cytotoxicity and antiviral activity of electrochemical-synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57.
- Jannat, K.; Paul, A.K.; Bondhon, T.A.; Hasan, A.; Nawaz, M.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Pereira, M.D.L.; et al. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics 2021, 13, 1895.
- Sofy, A.R.; Hmed, A.A.; El Haliem, N.F.A.; Zein, M.A.-E.; Elshaarawy, R.F. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261.
- Li, Y.; Lin, Z.; Xu, T.; Wang, C.; Zhao, M.; Xiao, M.; Wang, H.; Deng, N.; Zhu, B. Delivery of VP1 siRNA to inhibit the EV71 virus using functionalized silver nanoparticles through ROS-mediated signaling pathways. RSC Adv. 2017, 7, 1453–1463.
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048.
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Hua, L.; Wang, H.; Xia, H.; Zhu, B. Silver Nanoparticle based codelivery of ooeltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl. Mater. Interfaces 2016, 8, 24385–24393.
- Bartczak, D.; Muskens, O.L.; Sanchez-Elsner, T.; Kanaras, A.G.; Millar, T.M. Manipulation of in vitro angiogenesis using peptide-coated gold nanoparticles. ACS Nano 2013, 7, 5628–5636.
- El-Gaffary, M.; Bashandy, M.M.; Ahmed, A.R.; El-Borady, O.M. Self-assembled gold nanoparticles for in-vitro inhibition of bovine viral diarrhea virus as surrogate model for HCV. Mater. Res. Express 2019, 6, 075075.
- Sametband, M.; Shukla, S.; Meningher, T.; Hirsh, S.; Mendelson, E.; Sarid, R.; Gedanken, A.; Mandelboim, M. Effective multi-strain inhibition of influenza virus by anionic gold nanoparticles. Med. Chem. Comm. 2011, 2, 421–423.
- Fahmi, M.Z.; Sukmayani, W.; Khairunisa, S.Q.; Witaningrum, A.M.; Indriati, D.W.; Matondang, M.Q.Y.; Chang, J.-Y.; Kotaki, T.; Kameoka, M. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016, 6, 92996–93002.
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small 2020, 16, e1906206.
- Du, X.; Xiao, R.; Fu, H.; Yuan, Z.; Zhang, W.; Yin, L.; He, C.; Li, C.; Zhou, J.; Liu, G.; et al. Hypericin-loaded graphene oxide protects ducks against a novel duck reovirus. Mater. Sci. Eng. C 2019, 105, 110052.
- Donskyi, I.S.; Azab, W.; Cuellar-Camacho, J.L.; Guday, G.; Lippitz, A.; Unger, W.E.S.; Osterrieder, K.; Adeli, M.; Haag, R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale 2019, 11, 15804–15809.
- Heya, M.; David Torres-Hernández, Á.; Heya, M.S.; Torres Hernández, A.D.; Cordero Díaz, A.; García Coronado, P.L. Biomedical apllication of nanoformulated plant Diagnóstico de las dermatofitosis View project Biomedical apllication of nanoformulated plant View project Biomedical apllication of nanoformulated plant. Artic. Int. J. Pharm. Sci. Res. 2022, 13, 1000.
- Madureira, A.R.; Pereira, A.; Pintado, M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439.




