Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samara Testoni | + 1701 word(s) | 1701 | 2022-01-14 07:47:06 | | | |
| 2 | Yvaine Wei | + 342 word(s) | 2043 | 2022-01-25 01:55:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Testoni, S. Soil Colour and Plant-Wax Markers. Encyclopedia. Available online: https://encyclopedia.pub/entry/18716 (accessed on 07 February 2026).
Testoni S. Soil Colour and Plant-Wax Markers. Encyclopedia. Available at: https://encyclopedia.pub/entry/18716. Accessed February 07, 2026.
Testoni, Samara. "Soil Colour and Plant-Wax Markers" Encyclopedia, https://encyclopedia.pub/entry/18716 (accessed February 07, 2026).
Testoni, S. (2022, January 24). Soil Colour and Plant-Wax Markers. In Encyclopedia. https://encyclopedia.pub/entry/18716
Testoni, Samara. "Soil Colour and Plant-Wax Markers." Encyclopedia. Web. 24 January, 2022.
Copy Citation
Most cases involving soil in criminal investigations in Brazil have focused on the chemical and mineralogical analyses of soil fractions without including the organic matter. The organic fraction contains plant-wax markers which may be useful to “fingerprint” forensic soils due to their chemical diversity, relative longevity and resistant nature.
forensic
gas chromatography
n-alkanes
alcohols
1. Introduction
In forensic science, soil is an important form of trace evidence and can be used to test for potential links between soil on a questioned item and a potential source location. Since traces of soil can readily become trapped on the underside of footwear and on vehicle tires, soil has long been considered to be a potential source of forensic information [1][2][3][4][5][6]. Provided that a well-tested and robust method (with chemical, physical, mineralogical and organic data) for the characterisation of the samples is available and used appropriately, a potential link can be tested and established between a suspect and a crime scene [3][4].
Inorganic soil analyses have been successfully used in forensic investigations [3][4][7][8][9][10][11], and organic approaches have less frequently been employed. Many soils have less than 5% (w/w) of organic matter content, particularly tropical and subtropical soils, and this has expressive effects on the biological, chemical and physical characteristics of the soil, even if it is present at reduced contents. Furthermore, this material can be used successfully in forensic studies, as organic matter is an inherent part of the soil, that is, for a given material to be considered as soil, it must have a minimum level of organic material, in addition to containing the inorganic mineral fractions (sand, silt and clay) in different proportions. As the concentration of organic matter varies with the soil concentration, dilutions of the organic matter content depend on the amount of soil sampled at the crime scene, such as the amount found on the sole of shoes and on the vehicle tires [3][4][12][13].
Traditional forensic approaches in Brazil have focused on the determination of the total elemental content and the physical fractionation of the soil [14]. In an alternative forensic approach, the composition of soil organic matter is determined by pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) [15]. Py–GC/MS analyses allow a qualitative investigation of the nature of organic materials and are efficient in identifying different compounds at the molecular level [16]. Soil organic biomarkers have received limited attention in forensic soil science, with only a few studies worldwide [17][18][19][20]. The relative abundance of organic compounds can vary considerably between soil samples only a short distance apart [13][21]. In most soils, especially in the topsoil, the organic compounds which have originated from the surface wax of plants are abundant. These compounds enter into the soil as fallen plant leaves and plant litter such as seeds, flowers and stems. Since plant roots have low concentrations of plant-wax compounds, they generally make a small contribution to the total content of these compounds in soils [4][18]. The most common and widely studied wax-marker compound class is the hydrocarbons, including n-alkanes, and the free and esterified long-chain fatty alcohols (high molecular-weight, straight-chain primary alcohols, derived from natural fats and oils) and fatty acids (carboxylic acids with a long aliphatic chain, containing a hydrocarbon chain and a terminal carboxyl group) [17][22][23][24] can also be used.
Forensic studies using plant-wax compounds have been tested mainly under temperate conditions, over a wide range of both rural and urban areas [17][20]. Under tropical and subtropical conditions, the potential of the use of plant-wax biomarkers in a forensic context is still unknown. The soil has a unique and ubiquitous nature given its physical, chemical, biological and mineralogical characteristics, which allow it to be differentiated between the most varied environments. The uniqueness of the soil in regard to its characteristics makes it a powerful source of information in forensic investigations, because, due to its variability, it is possible to discriminate and associate it with a specific individual or object related to a crime scene. In tropical and subtropical conditions, it is observed that inorganic mineral fractions are more frequently used to investigate traces of soil sampled at crime scenes, possibly due to several factors, such as the heterogeneous nature of soils under different climatic conditions which leads, for example, to the wide variations of the organic matter decomposition rates in relation to soils from temperate regions.
2. Soil Sites and Sampling
Two areas from the Curitiba municipality and two areas from the Colombo municipality, Paraná State, in the South of Brazil, were selected: the Santa Cândida and Boa Vista neighbourhoods of Curitiba and the Guarani and Guaraituba neighbourhoods of Colombo, located in the Metropolitan Region of Curitiba (Figure 1 and Figure 2). All soils were classified as inceptisol according to U.S. Soil Taxonomy [25] and as cambissolo háplico according to the Brazilian Soil Classification System [26].
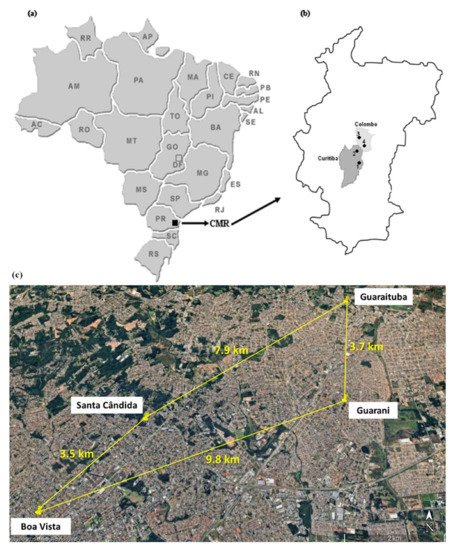
Figure 1. (a) Schematic figure of Brazil highlighting the Paraná State (PR) and the Curitiba Metropolitan Region (CMR); (b) two selected municipalities within the CMR; and (c) linear distance between the sampled sites (site 1—Santa Cândida neighbourhood; site 2—Boa Vista neighbourhood, Guarani neighbourhood, Guaraituba neighbourhood).

Figure 2. Sampling locations within Curitiba and Colombo municipalities, State of Paraná, Brazil. Photographs located in four separate urban areas: Santa Cândida—Curitiba (a); Boa Vista—Curitiba (b); Guarani—Colombo (c); Guaraituba—Colombo (d). Source: modified from (Testoni et al., 2019).
Locations which were not currently built over were selected across each urban area, and at each site four surface soils were sampled from the corners of a 1.5 m square (0–0.5 cm) quadrant in two replicates (Table 1).
Table 1. Soil sample characteristics.
| UTM (22J) | |||||
|---|---|---|---|---|---|
| Sample | Position 1 | Place | E-W | N-S | Parent Material |
| Site 1 | |||||
| 1 | Bottom right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 2 | Bottom left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 3 | Top right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 4 | Top left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 5 | Bottom right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 6 | Bottom left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 7 | Top right | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 8 | Top left | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 9 | Footprint/toes | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 10 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 11 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 12 | Footprint/toes | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| 13 | Footprint/heel | Santa Cândida | 678,203 | 7,192,226 | Claystone |
| Site 2 | |||||
| 14 | Bottom right | Guarani | 682,747 | 7,192,633 | Limestone |
| 15 | Bottom left | Guarani | 682,747 | 7,192,633 | Limestone |
| 16 | Top right | Guarani | 682,747 | 7,192,633 | Limestone |
| 17 | Top left | Guarani | 682,747 | 7,192,633 | Limestone |
| 18 | Bottom right | Guarani | 682,747 | 7,192,633 | Limestone |
| 19 | Bottom left | Guarani | 682,747 | 7,192,633 | Limestone |
| 20 | Top right | Guarani | 682,747 | 7,192,633 | Limestone |
| 21 | Top left | Guarani | 682,747 | 7,192,633 | Limestone |
| 22 | Footprint/toes | Guarani | 682,747 | 7,192,633 | Limestone |
| 23 | Footprint/heel | Guarani | 682,747 | 7,192,633 | Limestone |
| 24 | Footprint/heel | Guarani | 682,747 | 7,192,633 | Limestone |
| 25 | Footprint/toes | Guarani | 682,747 | 7,192,633 | Limestone |
| Site 3 | |||||
| 26 | Bottom right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 27 | Bottom left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 28 | Top right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 29 | Top left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 30 | Bottom right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 31 | Bottom left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 32 | Top right | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 33 | Top left | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 34 | Footprint/toes | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 35 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 36 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 37 | Footprint/toes | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 38 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| 39 | Footprint/heel | Guaraituba | 683,189 | 7,195,492 | Limestone |
| Site 4 | |||||
| 40 | Bottom right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 41 | Bottom left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 42 | Top right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 43 | Top left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 44 | Bottom right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 45 | Bottom left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 46 | Top right | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 47 | Top left | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 48 | Footprint/toes | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 49 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 50 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 51 | Footprint/toes | Boa Vista | 676,186 | 7,190,193 | Claystone |
| 52 | Footprint/heel | Boa Vista | 676,186 | 7,190,193 | Claystone |
1 Footprint heel and toes are simulated trace samples, collected at the centre of the sampling quadrant. Santa Cândida and Boa Vista are neighbourhoods of the Curitiba municipality (State of Paraná), Guarani and Guaraituba are neighbourhoods of the Colombo municipality (Curitiba Metropolitan Region).
3. Soil Colour Analysis
The samples collected in the Guarani neighbourhood (Table 1) presented the lowest values for the E4/E6 ratio (Figure 3a). The E4/E6 ratio is related to the condensation degree of the aromaticity or aliphaticity and the molecular weight of organic compounds [27][28][29]. Higher E4/E6 ratios (Boa Vista neighbourhood) are generally associated with more aliphatic and low-molecular-weight structures [30], with branched and straight-chain carbons [31][32]. The greater presence of vegetation with aliphatic groups (less humified) in Boa Vista could also be observed in the greater reflectance at 725–720 nm (red region) (Figure 3b), typical of long aliphatic chains with C–C and C–H bonds [31]. The RGB reflectance of the visible spectrum (500 to 720 nm) may be attributed to less aromatic chemical structures. These relationships obtained through colour spectroscopy and the qualitative aspects of the organic matter may be related to the nature of the residue of plants present in the soil [30][33].
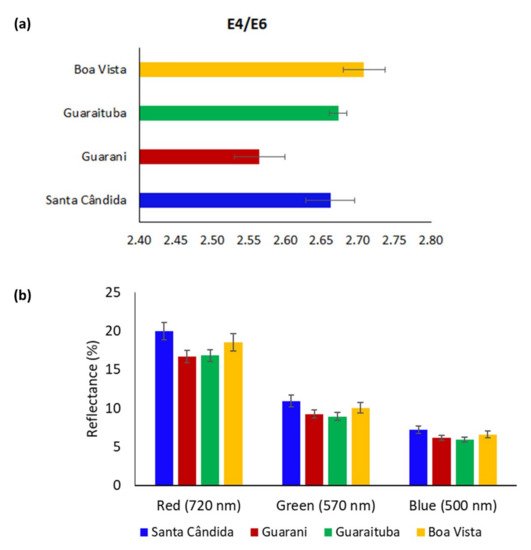
Figure 3. Average of soil-colour parameters for the four sites: (a) E4/E6 ratio (absorbance at 465/absorbance at 665 nm) and (b) RGB (red, green, blue) data obtained from the reflectance values of the visible spectra at 720, 570 and 500 nm wavelengths, respectively (Santa Cândida—n = 13 samples (Table 1) × 3 replicates = 39; Guarani—n = 12 samples × 3 replicates = 36; Guaraituba—n = 14 samples × 3 replicates = 42; Boa Vista—n = 13 samples × 3 replicates = 39).
At all the sites there was a predominance of odd long-chain alkanes (C29, C31 and C33); on the other hand, the values of C35 were low (Figure 4a). The hydrocarbons, including n-alkanes, are the most common and widely studied wax compounds [22][34][35]. The provenance of these compounds is mainly from the wax of the vegetation that enters into the soil as fallen leaves and other plant litter such as seeds, flowers and stems [12]. The n-alkanes, with odd-numbered carbon chains, are the predominant markers found in cuticular wax, especially the C27, C29, C31 and C33 alkanes [24][36][33], with C29 and C31 being the dominant compounds (Figure 4a).
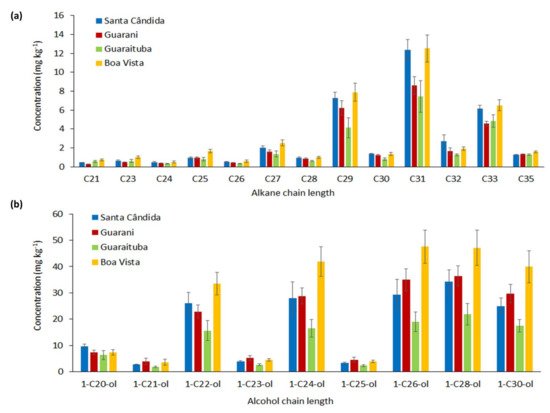
Figure 4. Mean (± standard deviation) n-alkane (a) and alcohol (b) concentrations for the four sites (Santa Cândida—n = 13 samples (Table 1) × 3 replicates = 39; Guarani—n = 12 samples × 3 replicates = 36; Guaraituba—n = 14 samples × 3 replicates = 42; Boa Vista—n = 13 samples × 3 replicates = 39).
C27, C29 and C31 alkane content is commonly found in the same corresponding plants, which explains the greater presence of these long-chain waxes in all the soils from the sites studied (Figure 4a). However, the compound concentrations in the plant may vary according to the photosynthetic pathways [37]. In some studies, it has been postulated that C31 represents grass input, while C27 and C29 represent input from trees and shrubs [17][22][23][24]. The input of different plant species at various stages of decomposition as a result of more intensive human influence is reflected in the higher concentrations of these long-chain alkanes at the Boa Vista site (Figure 4a). The lower concentrations of n-alkanes at the Guarani site (Figure 4a) may be associated with the low E4/E6 ratios (Figure 3a), which indicate the small input of more condensed chemical structures.
The consequent soil wax concentrations were also a result of the climatic conditions, reflecting the faster biosynthesis of these compounds under tropical and subtropical conditions [38] than under temperate conditions. The biodegradation of n-alkanes, for example, occurs at mesophilic temperatures [25]. Higher temperatures and increased rainfall were commonly found in Brazil, compared to the studies carried out in a temperate climate such as in the UK [39][40].
All the variables obtained in the study (spectral colour, alkanes and alcohols) were explored using principal component analysis (PCA) (Figure 5). The cumulative variance of the principal components one and two (PC1 and PC2) was high. The samples from the Guaraituba neighbourhoods were grouped well in the PCA (Figure 5a), with a related Bray–Curtis clustering of similarity (6a) based on n-alkanes and fatty alcohols. The use of plant-wax markers in discriminating between soils/sediments from different plant communities has been clearly demonstrated on UK soils under temperate conditions [4][36][39].
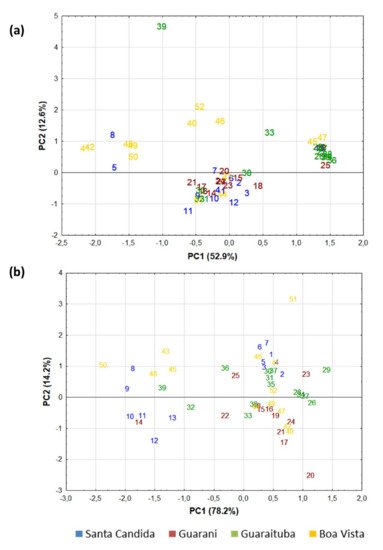
Figure 5. Principal Component Analysis (PCA) of the soil samples analysed based on n-alkanes and fatty alcohols (a) and spectral colour data (b).
4. Conclusions
Both the soil colour and the composition of the plant-wax markers were highly variable at medium (metres) and short distances (centimetres). Under the environmental conditions of the present study (urban area with a subtropical climate), the composition of the wax markers was different in samples collected side-by-side (for example, the sole of a suspect’s boot). The composition of the biomarkers was very sensitive to local variations under subtropical conditions and in areas under intense urban use. Under these conditions, biomarkers may be used in real crime scenes on a centimetre scale of variation or when using average values from a large number of soil samples. The analysis of alkanes was more discriminating compared with alcohols. Consideration should be given to the spatial scale of the variability of the methods used in any investigation, as it is important in the evaluation of potential linkages with a particular location, such as at a crime scene. This is why two or more analytical methods are often adopted in a case to avoid potential false exclusions or false associations.
References
- Morgan, R.M.; Bull, P.A. Data Interpretation in Forensic Sediment and Soil Geochemistry. Environ. Forensics 2006, 7, 325–334.
- Ritz, K.; Dawson, L.; Miller, D. Criminal and Environmental Soil Forensics; Springer: New York, NY, USA, 2008.
- Dawson, L.A.; Hillier, S. Measurement of soil characteristics for forensic applications. Surf. Interface Anal. 2010, 42, 363–377.
- Fitzpatrick, R.W.; Raven, M.D. Guidelines for Conducting Criminal and Environmental Soil Forensic Investigations: Version 7.0.076; Acid Sulfate Soils Centre: Adelaide, Australia, 2012.
- Murray, K.R.; Fitzpatrick, R.W.; Bottrill, R.S.; Berry, R.; Kobus, H. Soil transference patterns on bras: Image processing and laboratory dragging experiments. Forensic Sci. Int. 2016, 258, 88–100.
- Chauhan, R.; Kumar, R.; Diwan, P.; Sharma, V. Thermogravimetric analysis and chemometric based methods for soil examination: Application to soil forensics. Forensic Chem. 2020, 17, 100191.
- Nakai, I.; Furuya, S.; Bong, W.; Abe, Y.; Osaka, K.; Matsumoto, T.; Itou, M.; Ohta, A.; Ninomiya, T. Quantitative analysis of heavy elements and semi-quantitative evaluation of heavy mineral compositions of sediments in Japan for construction of a forensic soil database using synchrotron radiation X-ray analyses. X-ray Spectrom. 2014, 43, 38–48.
- Melo, V.F.; Testoni, S.A.; Dawson, L.; de Lara, A.G.; da Silva Salvador, F.A. Can analysis of a small clod of soil help to solve a murder case? Sci. Justice 2019, 59, 667–677.
- Testoni, S.A.; Melo, V.F.; Dawson, L.A.; Salvador, F.A.D.S.; Kunii, P.A. Validation of a Standard Operating Procedure (SOP) for Forensic Soils Investigation in Brazil. Revista Brasileira de Ciência do Solo 2019, 43, e0190010.
- Prandel, L.; Melo, V.F.; Testoni, S.A.; Brinatti, A.M.; Saab, S.D.C.; Dawson, L.A. Spectroscopic techniques applied to discriminate soils for forensic purposes. Soil Res. 2020, 58, 151.
- Dawson, L.A.; Mayes, R.W. Criminal and Environmental Soil Forensics: Soil as Physical Evidence in Forensic Investigations, Introduction to Environmental Forensics, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 457–486.
- Dawson, L. Soil organic characterisation in forensic case work. J. Int. Geosci. 2017, 40, 157–165.
- Melo, V.; Mazzetto, J.M.; Dieckow, J.; Bonfleur, E.J. Factor analysis of organic soils for site discrimination in a forensic setting. Forensic Sci. Int. 2018, 290, 244–250.
- Mazzetto, J.M.; Melo, V.F.; Bonfleur, E.; Vidal-Torrado, P.; Dieckow, J. Potential of soil organic matter molecular chemistry determined by pyrolysis-gas chromatography/mass spectrometry for forensic investigations. Sci. Justice 2019, 59, 635–642.
- Schellekens, J.; Almeida-Santos, T.; Macedo, R.S.; Buurman, P.; Kuyper, T.W.; Vidal-Torrado, P. Molecular composition of several soil organic matter fractions from anthropogenic black soils (Terra Preta de Índio) in Amazonia—A pyrolysis-GC/MS study. Geoderma 2017, 288, 154–165.
- Dawson, L.A.; Towers, W.; Mayes, R.W.; Craig, J.; Väisänen, R.K.; Waterhouse, E.C. The use of plant hydrocarbon signatures in characterizing soil organic matter. Geol. Soc. Spec. Publ. 2004, 232, 269–276.
- Carvalho, Á.; Ribeiro, H.; Mayes, R.; Guedes, A.; Abreu, I.; Noronha, F.; Dawson, L. Organic matter characterization of sediments in two river beaches from northern Portugal for forensic application. Forensic Sci. Int. 2013, 233, 403–415.
- Madureira-Carvalho, Á.; Ribeiro, H.; Newman, G.; Brewer, M.J.; Guedes, A.; Abreu, I.; Noronha, F.; Dawson, L. Geochemical analysis of sediment samples for forensic purposes: Characterisation of two river beaches from the Douro River, Portugal. Aust. J. Forensic Sci. 2018, 52, 222–234.
- Mcculloch, G.; Dawson, L.A.; Ross, J.M.; Morgan, R.M. The discrimination of geoforensic trace material from close proximity locations by organic pro fi ling using HPLC and plant wax marker analysis by GC. Forensic Sci. Int. 2018, 288, 310–326.
- Demanèche, S.; Schauser, L.; Dawson, L.; Franqueville, L.; Simonet, P. Microbial soil community analyses for forensic science: Application to a blind test. Forensic Sci. Int. 2016, 270, 153–158.
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry-an overview of indicators of organic matter sources and dia-genesis in lake sediments. Org. Geochem. 1993, 20, 867–900.
- Meyers, P.A. Application of organic geochemistry to paleolimnological reconstruction: A summary of examples from the Laurention Great Lakes. Org. Geochem. 2003, 34, 261–289.
- Schellekens, J.; Buurman, P.; Pontevedra-Pombal, X. Selecting parameters for the environmental interpretation of peat mo-lecular chemistry–A pyrolysis-GC/MS study. Org. Geochem. 2009, 40, 678–691.
- Dove, H.; Mayes, R.; Freer, M. Effects of species, plant part, and plant age on the n-alkane concentrations in the cuticular wax of pasture plants. Aust. J. Agric. Res. 1996, 47, 1333–1347.
- Soil Survey Staff. Keys to Soil Taxonomy; Natural Resources Consersation Services: Washington, DC, USA, 2014.
- Dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Empresa Brasileira de Pesquisa Agropecuária: Brasilia, Brasil, 2018.
- Chauhan, R.; Kumar, R.; Sharma, V. Soil forensics: A spectroscopic examination of trace evidence. Microchem. J. 2020, 139, 74–84.
- Zalba, P.; Amiotti, N.M.; Galantini, J.A.; Pistola, S. Soil Humic and Fulvic Acids from Different Land-Use Systems Evaluated by E4/E6 Ratios. Commun. Soil Sci. Plant Anal. 2016, 47, 1675–1679.
- Oktaba, L.; Odrobińska, D.; Uzarowicz, Ł. The impact of different land uses in urban area on humus quality. J. Soils Sediments 2018, 18, 2823–2832.
- Chauhan, R.; Kumar, R.; Kumar, V.; Sharma, K.; Sharma, V. On the discrimination of soil samples by derivative diffuse reflectance UV–vis-NIR spectroscopy and chemometric methods. Forensic Sci. Int. 2020, 319, 110655.
- Senesi, N.; Sipos, S. Molecular-weight distribution, analytical and spectroscopic characterization of humic fractions sequentialy isolated by organic-solvents from a brown coal acid. Org. Geochem. 1985, 8, 157–162.
- Mbarek, B.H.; Gargouri, K.; Mbadra, C.; Chaker, R.; Souidi, Y.; Abbas, O.; Baeten, V.; Rigane, H. Change and spatial variability of soil organic matter humification after long-term tillage and olive mill wastewater application in arid regions. Soil Res. 2020, 58, 388.
- Sharma, V.; Chauhan, R.; Kumar, R. Spectral characteristics of organic soil matter: A comprehensive review. Microchem. J. 2021, 171, 106836.
- Mayes, R.W.; Macdonald, L.M.; Ross, J.M.; Dawson, L.A. Discrimination of Domestic Garden Soils Using Plant Wax Compounds as Markers. In Criminal and Environmental Soil Forensics; Ritz, K., Dawson, L.A., Miller, D., Eds.; Springer: Cham, Switzerland, 2008; pp. 463–476.
- Sachse, D.; Billault, I.; Bowen, G.J.; Chikaraishi, Y.; Dawson, T.E.; Feakins, S.J.; Freeman, K.H.; Magill, C.R.; McInerney, F.A.; van der Meer, M.T.; et al. Molecular Paleohydrology: Interpreting the Hydrogen-Isotopic Composition of Lipid Biomarkers from Photosynthesizing Organisms. Annu. Rev. Earth Planet. Sci. 2012, 40, 221–249.
- McCulloch, G.; Dawson, L.; Brewer, M.; Morgan, R. The identification of markers for Geoforensic HPLC profiling at close proximity sites. Forensic Sci. Int. 2017, 272, 127–141.
- Vogts, A.; Moossen, H.; Rommerskirchen, F.; Rullkötter, J. Distribution patterns and stable carbon isotopic composition of alkanes and alkan-1-ols from plant waxes of African rain forest and savanna C3 species. Org. Geochem. 2009, 40, 1037–1054.
- Holtvoeth, J.; Rushworth, D.; Copsey, H.; Imeri, A.; Cara, M.; Vogel, H.; Wagner, T.; Wolff, G.A. Improved end-member characterisation of modern organic matter pools in the Ohrid Basin (Albania, Macedonia) and evaluation of new palaeoenvironmental proxies. Biogeosciences 2016, 13, 795–816.
- Smith, D.G.; Mayes, R.W.; Raats, J.G. Effect of species, plant part, and season of harvest on n-alkane concentrations in the cuticular wax of common rangeland grasses from southern Africa. Aust. J. Agric. Res. 2001, 52, 875–882.
- Cheng, L.; Shi, S.; Li, Q.; Chen, J.; Zhang, H.; Lu, Y. Progressive Degradation of Crude Oil n-Alkanes Coupled to Methane Production under Mesophilic and Thermophilic Conditions. PLoS ONE 2014, 9, e113253.
More
Information
Subjects:
Soil Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
848
Revisions:
2 times
(View History)
Update Date:
25 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




