Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samuele Ciceri | + 4868 word(s) | 4868 | 2021-12-20 07:47:07 | | | |
| 2 | Jessie Wu | Meta information modification | 4868 | 2022-01-24 02:31:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciceri, S. Applications of Lysozyme. Encyclopedia. Available online: https://encyclopedia.pub/entry/18652 (accessed on 12 January 2026).
Ciceri S. Applications of Lysozyme. Encyclopedia. Available at: https://encyclopedia.pub/entry/18652. Accessed January 12, 2026.
Ciceri, Samuele. "Applications of Lysozyme" Encyclopedia, https://encyclopedia.pub/entry/18652 (accessed January 12, 2026).
Ciceri, S. (2022, January 23). Applications of Lysozyme. In Encyclopedia. https://encyclopedia.pub/entry/18652
Ciceri, Samuele. "Applications of Lysozyme." Encyclopedia. Web. 23 January, 2022.
Copy Citation
Lysozyme (or muramidase or N-acetylmuramic acid hydrolase E.C. 3.2.1.17) is a protein that exerts its enzymatic activity through the hydrolysis of the β-1,4-glycosidic bonds between N-acetylmuramic acid (NAM) and N-acetylglucosamide (NAG) in the polysaccharide backbone of the peptidoglycans of the Gram-positive bacterial cell wall. Peptidoglycan is composed of polysaccharide chains cross-linked by short peptides. The polysaccharide chains contain alternate units of NAM and NAG.
lysozyme
innate immunity
antimicrobial
1. Applications of Lysozyme
The large number of investigated sources of different types of lysozymes can be explained by the many applications in medicine, cosmetics, the food industry, and agriculture. The wide spectrum of applications depends not only on its antibacterial activity, but also on the inactivation of certain viruses and fungi.
The antibacterial activity against Gram-positive bacteria has been explained by the lysozyme enzymatic action on the peptidoglycans present in the cell wall. The peptidoglycans present in the inner membrane of Gram-negative bacteria are shielded by a lipidic outer membrane, but the lysozyme shows, even if weakly, to be active. Some authors explained this activity, proposing that the antibacterial mechanism of action is independent of its enzymatic activity, also in the case of Gram-positive bacteria. The role of the lysozyme, according to this hypothesis, is the removal of the cell wall of the bacteria previously killed by antimicrobial polypeptides. Ibrahim et al., in 2001 [1], showed that the catalytically inactive mutant of the hen egg white lysozyme was as bactericidal as the wild -type lysozyme against S. aureus and B. subtilis. An opposite opinion was suggested in the same year by Masschlck et al. [2], who observed that a high pressure treatment, in the presence of lysozyme, sensitized a series of Gram-negative bacteria; the denaturation of lysozyme, by heat treatment, fully eliminated the bactericidal effect observed under high pressure conditions, while a partially denatured lysozyme maintained its activity. The bactericidal effect, due to the high-pressure treatment, was observed also in the case of two peptides, devoid of enzymatic activity, obtained from lysozyme: the authors ascribed these results to the cationic nature and the increased hydrophobicity of the chains.
The role of cationic peptide chains (the depolarization and permeabilization of membranes) was discussed in an article published in 2004 [3], together with the hypothesis of the indirect bactericidal action of lysozyme: the cationic peptide can behave as antibacterial by activating an autolytic wall muramidase of bacteria (a phenomenon defined as “Trojan horse”), resulting in bacteriolysis.
The action mechanism of lysozyme was studied also in vivo to verify if its activity depends or not on the muramidase action. From the observed results in transgenic mice deficient in lysozyme or expressing a muramidase-deficient lysozyme transgene, the authors concluded that lysozyme kills bacteria independently of its muramidase activity [4].
The cationic nature of the lysozyme chain was hypothesized to be the cause of its fungicidal activity. The ionic interactions between the cationic peptide and the anionic structures in the microbial cell wall can result in the damage to the cell wall, which is disrupted by a subsequent event, such as the exposure to salt and detergent by the effect of osmotic pressure [5].
In an article published in 1999, the presence of a protein with the N-terminal 15 amino acids sequence identical to the human urinary lysozyme C in preparations of the β-subunit of human chorionic gonadotropin, was reported. The antiviral activity of this protein and of the lysozymes from chicken egg whites, from human milk, and from human neutrophils against HIV-1, was explained by the authors as being due to the degradation of viral polysaccharides [6].
The number of suggested potential mechanisms of action is as wide as the field of lysozyme applications against microorganisms, which differ greatly from one another.
2. Medical Applications
2.1. Skin Diseases
The milky juice of papaya fruits is a source of proteolytic enzymes, including lysozyme, and it is applied in surgery for the treatment of fistulas, cleaning wounds from necrotized tissues, and for skin grafting [7].
High antibacterial effects produced by both bacteriostatic and bactericidal pathways, including lysozyme activity, was demonstrated for the seed oil from Carthamus tinctorius (safflower) [8], in the management of skin injuries.
Staphylococcus aureus is the most common cause of primary and post-operative skin infections, and it has become increasingly resistant to antibiotics, such as methicillin and vancomycin. The use of lysozyme from egg whites and its dextran conjugate was investigated as an alternative topical ointment for the treatment of the infected skin of mice [9]. The two preparations were tested in vitro against S. aureus and E. coli. The results showed that both the lysozyme and lysozyme conjugate exhibited antibacterial activity against S. aureus, but only the lysozyme conjugate was active against E. coli. The activity of the conjugated lysozyme was explained by the authors by the strong surface activity which can enhance the lytic action of the enzyme toward the peptidoglycan layer in the inner membrane. The studies on mice also confirmed the improvement of the antibacterial activity of the lysozyme in wound healing, due to the conjugation with dextran. The activity of the dextran conjugated lysozyme was comparable with tetracycline, suggesting that lysozyme is a natural antimicrobial agent and a suitable replacement for synthetic antibiotic.
In a 2017 publication [10], a smart antimicrobial system, activated in the case of infection, based on elevated lysozyme activity, was presented. A synthesized N-acetyl chitosan was subjected to the lysozyme hydrolysis in artificial wound fluid, presenting N-acetylated chitooligosaccharides (COS). COS, by action of cellobiose dehydrogenase, afforded antimicrobial hydrogen peroxide (1 mM), which is able to inhibit the growth of E. coli and S. aureus (Figure 1).
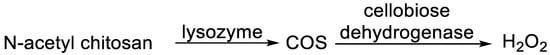
Figure 1. Antimicrobial system involving lysozyme.
A T4 lysozyme fused with a cellulose binding module was prepared and immobilized to a wound dressing gauze. The immobilized protein retained the bacterial activity against Gram-positive and Gram-negative bacteria. The unmodified T4 lysozyme could not bind to the gauze. The immobilized lysozyme can constitute an innovative strategy for producing antimicrobial wound dressing materials [11].
Acticoat, an antibacterial silver nanoparticle-loaded dressing, is a commonplace for the prevention of infection in burns and with open wound patients. The efficacy of this dressing against methicillin-resistant S. aureus (MRSA) was evaluated, investigating additives that can improve its activity [12]. The greatest reduction in bacterial survival was observed when Acticoat was soaked with a combination of 10% glycerol, lysozyme (1 mg/mL), and an antimicrobial peptide (bac8c, a truncated and modified bovine neutrophile peptide).
A promising preparation for the development of antibacterial wound dressing was obtained in 2018 [13], starting from hairy steric stabilized nanocrystalline cellulose (SNCC) functionalized with aldehyde groups; by the reaction of these groups, lysozyme or nisin was immobilized on cellulose. Lysozyme and nisin in free and immobilized forms were tested against B. subtilis and S. aureus. S. aureus is the bacterial species more commonly detected in infected wounds and B. subtilis is closely related to several animal pathogens, including B. cereus, which is associated with wound infections. Immobilized nisin showed to be active against S. aureus, whereas free nisin became ineffective against the growth of S. aureus after 24 h. Lysozyme was not effective against S. aureus, but the immobilized lysozyme was active against B. subtilis. The authors of the study suggest that the combination of antimicrobial agents immobilized onto SNCC can offer an effective broad spectrum antibacterial wound dressing.
Recently [14], the effect of wound moisture on wound healing was studied considering the moisture balance of a polyurethane foam dressing. A moisture balanced antibacterial dressing was constructed by loading lysozyme onto a polyurethane foam dressing, by means of dopamine adsorption. The prepared dressing experiment in wound healing in infected mice provided the appropriate wound moisture and at the same time prevented bacterial infections.
The most common skin disorder is the acne vulgaris caused by Propionobacterium acnes. The use of lysozyme-shelled microbubbles (MBs) and ultrasound-mediated Lys-MBs cavitation against P. acnes, in vitro and in vivo, aimed to reduce the dose and the duration of antibiotic therapy, was investigated [15]. The results of the study showed that the combined Lys-MBs and ultrasound significantly reduced the treatment duration and inhibited P. acnes-induced skin diseases.
A different approach to the control of P. acnes by lysozyme was proposed in 2018. Bacteriocin AS-48 is a 70-amino acid residue circular peptide produced by different Enterococcus species, endowed with bactericidal activity on many Gram-positive and Gram-negative bacteria. The effectiveness against P. acnes by AS-48 alone, and in combination with lysozyme, was examined using a range of microscopy and bioassay techniques. The improvements of the action of AS-48 through the combination with lysozyme showed that these two natural compounds are promising candidates against dermatological diseases, such as acne vulgaris [16].
The use of a lysozyme gel formulation in the disinfection of the skin, during pre- and post-surgery, for facial care and the care of hands, feet, and nails, was reported in a 2013 U.S. Patent [17]. The gelled lysozyme was prepared by the addition of water to a suspension of lysozyme in alcohol, without the addition of other gelling substances. The formulation, retaining the enzymatic activity, was successfully used for local applications in the pre- and post-operative therapy of phlebopathic patients.
A polyethylene-based material loaded with an antibiotic is often used as a surgical sealant, but the developed drug resistance prompted the development of a variety of bioactive molecules modified by a PEG-based hydrogel. The antibacterial activity of lysozyme prompted the development of a PEG-lysozyme injectable sealant. A four-arm PEG suitably functionalized at each terminal arm was linked to lysozyme through its amine groups. It was observed that the hydrogel sealed gas or blood leakage in a rabbit trachea, and it could close the transmural left ventricular wall defect. The bacteriostatic activity was demonstrated against S. aureus and E. coli [18].
2.2. Medical Devices
Bone tissue engineering plays a crucial role in regenerating defective or lost tissue. The selection of material is important since it is necessary to consider the biological properties of living cells and the physicochemical properties of the materials. A silica precursor was blended with a SiO2-CaO-P2O5 mesoporous bioactive glass solution. Lysozyme was encapsulated into the scaffold, which exhibited an ability to delay the pH-triggered lysozyme release endowed with antibiotic activity [19].
A silicon rubber film modified with N-vinyl caprolactam was prepared with the aim to reversibly load and release lysozyme for medical devices. The enzymatic activity was assessed against Micrococcus lysodeikticus [20].
Pelvic organ prolapse is a benign post-partum gynecological disease which seriously affects the quality of life of middle-aged and elderly women. Mesh, mainly polypropylene, is a material used for pelvic floor reconstruction. The postoperative complications prompt to find alternative materials. In a recent article [21], lysozyme and collagen were alternatively deposited on silk fibroin and nylon 6 composite nanofibrous mats through a layer-by-layer self-assembly method. Silk fibroin was chosen for its biological compatibility, biodegradability, and low immunogenicity. The poor mechanical properties of silk fibroin nanofibers were overcome by blending silk fibroin with nylon 6, a synthetic polymer with excellent mechanical strength. These mats showed an over 98% reduction in the viable count of S. aureus. The less encouraging results against E. coli can be explained because lysozyme is less sensitive to Gram-negative bacteria.
The immobilization of lysozyme onto woven or knitted PET [22], decreased the adhesion of vein catheter-isolated Staphylococcus epidermis by 6- to 8-fold and of S. aureus by 11- to 12-fold, while the Gram-negative E. coli showed a decrease of 3- to 4-folds. Immobilized lysozyme on PET can inhibit the graft-associated infection.
The oral healthcare of aged people is an issue of great interest and importance due to ageing societies. The development of self-cleaning materials is closely related to the adsorption phenomena on both dental material and teeth of antibacterial agents. These agents can be synthetic compounds or naturally secreted substances, such as lysozyme. The quartz crystal microbalance method associated with dissipation monitoring is a technique suitable for sensing the “softness”, or viscoelastic properties, of the adsorbed layer. Through this method, the adsorption of lysozyme on the Au sensor, SiO2 sensor, or TiO2 sensor, were studied at different pH conditions and salt concentrations [23]. The affinity of lysozyme was in the order of Au > SiO2 > TiO2 sensors. The pH-dependent charge of oxide surfaces showed to influence the lysozyme adsorption, suggesting that the nature of the adsorption on the oxide surface was electrostatic.
The osteointegration of dental implants with surrounding tissue and the presence of infection at the implant site are critical factors in the success of dental implants.
Graphene oxide, a nanosheet with a thickness of several atoms, has a large surface area and it is rich in functional groups. It possesses an appreciable ability to kill Gram-positive and Gram-negative bacteria, due to its behavior that resembles a nano knife being able to penetrate and cut the cell membrane. However, this nonspecific mechanism also affects other cells, leading to a biotoxicity strictly related to the concentration. In 2020 [24], graphene oxide was integrated with lysozyme and tannic acid into a thin coating using a layer-by-layer method. The tannic acid extracted from plants has antibacterial properties and strong osteogenesis, due to its binding to calcium ions. Subsequently, the new coating was characterized by the beneficial effects of the integrated components. The layer-by-layer method allowed us to overcome the concentration-dependent graphene oxide toxicity because a very low amount was required to cover the entire substrate. The efficacy of this coating was demonstrated against the Gram-positive S. aureus and the Gram-negative E. coli.
A lysozyme coating was proposed again in 2020 [25], with the aim to improve the corrosion resistance of new orthodontic composite arch wires (CAWs). The CAWs are made of a nickel and titanium alloy and stainless steel produced by laser welding, with copper serving as the interlayer. The copper interlayer can be damaged in the corrosive oral environment. The lysozyme coated arch wires were prepared using a liquid phase deposition with different concentrations of the enzyme. The corrosion behaviors of the CAWs and their antibacterial performance were examined: the composite arch wires coated with 20 g/L of lysozyme showed fewer corrosion pit and less corrosion depth relative to the uncoated CAWs. The concentration of 40 g/L of lysozyme was identified as the ideal future clinical application by the results observed against S. aureus.
2.3. Biofilms
Biofilms are matrix-enclosed accumulations of microorganisms, such as bacteria, fungi, protozoa, and viruses. They are rarely composed of a single type of cells. The non-cellular components can be carbohydrates, proteins, lipids, lipoproteins, and lipopolysaccharides. The adhesion of microorganisms can be encountered in the food environment (food processing and packaging). Some of the nosocomial infections are caused by the use of biomedical devices on which the biofilm of Staphylococci bacteria grow. Frequently, the common surface cleaning procedures are not sufficient against mature biofilms. Biofilm structures cause the reduced response of bacteria to antibiotics and weakens the bactericidal actions of antimicrobial and sanitizing agents. Many efforts are focused on the identification of surfaces able to prevent the adhesion of microorganisms and, at the same time, exhibiting biocide properties.
In a 2004 U.S. Patent [26], the preparation method of a two-component composition of two anchor enzyme complexes was described. The first complex degrades the biofilm structures. For example, it contains alginate lyase to degrade the polysaccharide matrix in which Pseudomonas aeruginosa can grow and proliferate. The second anchor-enzyme complex contains the lysozyme necessary to lyse the bacteria within the biofilm. This composition can be utilized on implanted medical devices (catheters and cannulae) to control a variety of infections.
Enterococcus faecalis is a Gram-positive gastrointestinal commensal and a leading cause of nosocomial infections. E. faecalis infections are difficult to treat because it forms biofilms and is resistant to many antimicrobial agents. The effect of lysozyme (from chicken egg white and rhLys) on E. faecalis biofilm was studied in a 2019 patent and a kit with lysozyme was developed for treating such a bacterial infection [27].
In another case [28], with the aim to protect stainless steel surfaces, the lysozyme from a hen egg white and PEG were coated onto the surfaces. The surfaces coated with the enzyme displayed high antiadhesion activity toward Listeria ivanovii and a marked local biocide activity on Micrococcus luteus.
In another biofilm study, stainless steel surfaces were chosen as the starting material in order to obtain a material with a bactericidal function [29]. The surface was first activated by a biomimetic catechol (dopamine) anchor; the amino group of dopamine was treated with glutaraldehyde, a bifunctional linker. Chitosan was covalently immobilized by the reaction with glutaraldehyde. Finally, lysozyme (from the hen egg white) was conjugated to the chitosan (Figure 2). Chitosan is a well-known biopolymer endowed with antibacterial activities against bacteria, fungi, and algae [30]. The modified surface showed that the properties of both chitosan and lysozyme were preserved, and a substantial enhancement of the activity against bacterial adhesion and bactericidal efficiency against S. aureus were observed on the lysozyme-immobilized substrates relative to the chitosan-grafted substrates.
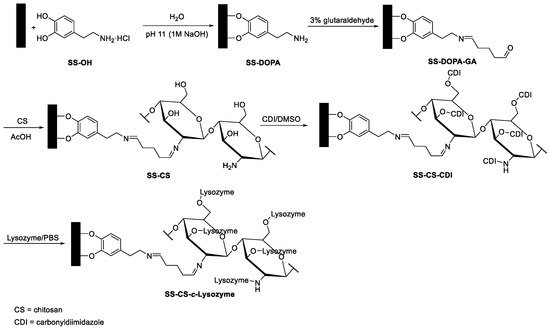
Figure 2. A schematic diagram illustrating the process of coupling dopamine, bifunctional cross-linker glutaraldehyde, chitosan, and lysozyme.
The immobilization of lysozyme on chitosan would offer a method for combating the biofilm-related contamination of stainless-steel implants and devices.
Streptococcus pneumoniae is a Gram-positive bacterium which causes many infections, ranging from the common and usually mild otitis and rhinosinusitis to invasive diseases, such as sepsis, pneumonia, and meningitis. Since it has become increasingly resistant to antibiotics, new antimicrobials have been developed, including the use of bacteriophages and some of their products. Two pneumococcal phage lysozymes (Cpl-1 and Cpl-7) were chosen with the aim to construct four dimeric proteins by shuffling their two functional domains and the linkers. The ability of these new enzymes to reduce pneumococcal biofilm formation and the killing activity against pneumococcal bacteria were studied in mice. One of them showed a greater bactericidal capacity than the natural Cpl-1, confirming the power of this new chimeric enzyme [31].
Another Streptococcus, S. mutans, causes the formation of a biofilm in the human oral cavity and tooth decay. A study was carried out to improve the activity of marine Arthobacter oxydans KQ11 dextranase mouthwash by the best combination of ZnSO4, lysozyme, citric acid, and chitosan. The optimized formula tested in marine dextranase mouthwash enhanced the inhibition of S. mutans [32].
Antibacterial photodynamic therapy is used for caries, endodontic disease, periodontitis, and peri-implantitis. This technique utilizes reactive oxygen species, such as singlet oxygen and free radicals via photosensitizers, to reduce bacterial infections. In order to overcome the narrow spectrum of excitation wavelengths to generate O2 of the commonly chosen photosensitizers, a novel Au nanocluster photosensitizer was proposed [33]. This novel photosensitizer is a lysozyme-gold nanocluster (AuNCs)–rose Bengal (RB) conjugate. This conjugate showed to effectively inhibit the growth of oral Gram-negative and Gram-positive bacteria when exposed to white light-emitting diode irradiation. The photoexcited Lys-AuNCs-RB successfully decreased the formation of S. mutans biofilm.
The formation of biofilm around implants is the primary cause of infections in the mucosa and bone adjacent to the implant. The incorporation of antibacterial compounds into the coating of dental implants is an effective strategy to prevent biofilm formation. Lysozyme was immobilized on titanium surfaces forming an initial functional layer. This base was subsequently coated with silver nanoparticles, chitosan, and hyaluronic acid via a layer-by-layer, self-assembly technique. The obtained surface was examined by SEM and X-ray photoelectron spectroscopy. The inhibition of the biofilm formation and the antibacterial activity were evaluated against S. aureus. The results showed that the modified Ti surfaces were effective in preventing bacteria colonization for 14 days, until mucosa healing [34].
The same techniques were followed, starting from a metal laser sintered Ti implant primed with phase-transited lysozyme, loading minocycline into polyelectrolyte multilayers, obtained, in turn, by the self-assembly of negative charged hyaluronic acid and positively charged chitosan, natural polysaccharides, non-toxic, and biodegradable [35]. The obtained modified titanium exhibited a strong antibacterial effect against the oral bacteria Streptococcus sanguis and Streptococcus gorgonii, two pathogenic species in the biofilm formation process.
Candida albicans is a yeast present in the oral cavity that can incorporate that form into biofilms on denture surfaces, leading to, in some cases, denture stomatitis. Lysozyme present in saliva (1–57 μg/mL) contributes to the control of oral microflora. In 2017, an in vitro study [36] investigated the effects of lysozyme on C. albicans biofilm formation. The results showed that at a low concentration (<30 μg/mL), lysozyme reduced the attached biomass, whereas with concentrations of >300 μg/mL, a pro-biofilm effect was observed.
Among the substances suitable for use as a raw material, for the preparation of surfaces effective for inhibiting biofilm formation, polyhydroxyalkanoate [37] was chosen to form sheets, onto which lysozyme was loaded. The maximum loading was 16.1 μg enzyme per 9.5 mm3 disks. These sheets, endowed with an effective inhibition for biofilm formation, can find an application for the fabrication of wound dressing in anti-biofilm treatments.
To remove bacteria biofilm, new ways to deliver antimicrobial agents in a sufficiently high concentration are required. Liposomes are considered as an attractive carrier for drug delivery for their potential to fuse with phospholipid membranes. The spontaneous fusion, resulting in payload loss, can cause a problem. In 2017 [38], a Chinese team proposed a novel lysozyme preparation as a gentamicin carrier. The antibiofilm activity of lysozyme, associated liposomal gentamicin, was tested against Pseudomonas aeruginosa, a Gram-negative human pathogen. This microorganism causes chronic pulmonary infections and is generally employed as a model organism for the investigation of biofilm. The study demonstrated that the positively charged lysozyme stabilized the negative-charged liposomes, and the lysozyme-associated liposomal gentamycin was more effective against the biofilms built by Gram-positive (S. aureus) and Gram-negative bacteria than lysozyme or gentamycin alone.
The lysozyme activity, in combination with cefepime or ceftazidime against the Pseudomonas aeruginosa biofilm, was recently studied [39]. The results showed that 50 times of the minimum inhibition concentrations (MICs) of 2 cephalosporins, in presence of lysozyme (30 μg/mL), significantly inhibited the 24 h-old biofilm that was formed, when compared with individual antibiotic treatment. The highest inhibitory effect (a 49.3% reduction) was observed with the combination of cefepime and lysozyme. This method can be applied to the eradication of P. aeruginosa biofilms from catheters, ocular lenses, intravascular devices, and ventilator tubes.
This synergistic effect against P. aeruginosa was also observed when a combination of ciprofloxacin (at sub MIC of 1.56 μg/mL) with lysozyme was applied. Indeed, in the case of the combination, a 56 ± 0.6% biofilm eradication was obtained, whereas a 40 ± 0.5% eradication was observed with ciprofloxacin, alone [40].
The co-administration of an antibiotic, clindamycin, or metronidazole, and rhLys, improved both the efficiency of the antibiotics and the lysozyme-driven biofilm degradation. This cotreatment was applied in an in vitro study on the biofilm of Gardnerella vaginalis, the bacterium involved in vaginal infections. Often, the antibiotic treatments are not resolutive and the patients suffer from recurrent infections, probably due to the inability of the antibiotics to reach the bacteria embedded inside the biofilm. The bactericidal and biofilm degradation effects, tested in this in vitro study, were greater than when lysozyme or antibiotics was tested alone [41].
The application of nanotechnology can overcome the difficulty exhibited by most antibiotics to cross the barriers of biofilm. In this context, C-dots, a new class of carbon nanomaterials, were synthesized from papaya leaf extracts and encapsulated with lysozyme into chitosan nanocarrier forming C-dots carriers (CDCs) [42]. The antibacterial activity of CDC was tested on B. subtilis (Gram-positive) and ampicillin-resistant E. coli (Gram-negative). The biofilm inhibition was studied on pellicle of B. subtilis. The combination of C-dots and lysozyme delivered by chitosan nanocarriers, inhibited the growth of both Gram-positive and Gram-negative bacteria, but more efficiently against Gram-positive bacteria. In addition, CDCs showed the capability not only to inhibit the biofilm formation, but also to eradicate the established biofilm. The authors are investigating the antibiofilm activity of CDCs on the surface of medical implants.
3. Lysozyme as Food Preservative
Food spoilage is the result of chemical, physical, or microbiological modifications that renders the food unacceptable. Microbial food spoilage occurs due to the growth of microorganisms (bacteria, fungi, and yeasts) in or on the food, and it is also the cause of most food-borne illnesses.
To overcome these undesired effects, manufactures and producers employ multiple strategies for food preservation, which include chemical, biological, and physical treatments. Lysozyme, in place of traditional antibiotics, can be utilized to preserve food and beverages in much the same manner, with reduced side effects practically limited to triggering adverse allergic reactions in susceptible individuals. For this reason, its presence in foods and beverages in many countries required the inclusion of egg allergens in labeling, when present in the final product (see, for example, Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 September 2011).
The use of lysozyme as a food additive is permitted in cheese, since it exerts an inhibitory effect against the growth of lactic acid bacteria involved in curd acidification and cheese ripening [43]. It also inhibits the growth of Clostridium tyrobutyricum that causes late blowing in hard and semi-hard cheese. In wine making, a maximum of 500 mg/L of lysozyme has been permitted, since 1996 (resolution OENO 10/97), because it helps to control malolactic fermentation by limiting lactic acid bacteria proliferation [44]. For unpasteurized beer, the concentration of 300 mg/L of lysozyme can reduce lactic acid beer spoilage bacteria, such as Pediococcus inopinatus, Lactobacillus brevis, Lactobacillus brevisimilis, and Lactobacillus lindneri [45].
To overcome the allergenic risks, due to the presence of lysozyme that can remain in food and beverages, different strategies were studied, including the immobilization through the Maillard reaction on different polysaccharides, to obtain films endowed with antimicrobial properties that can extend the shelf life of wrapped foods. For example, a bilayer edible film, based on chitosan and sodium alginate and incorporated with lysozyme, was demonstrated to be effective against fish spoilage bacteria, Pseudomonas fluorescens and Shewanella putrefaciens [46]; a lysozyme-chitosan composite film presented antimicrobial functions against both Gram-positive and Gram-negative representative bacteria (E. coli and S. faecalis) [47]; a lysozyme–dextran conjugate was effective against S. aureus and E. coli in a natural food system (cheese curd) [48]; a lysozyme–sodium alginate edible film was tested on two Gram-positive bacteria (i.e., Listeria innocua and Kocuria rhizophila) giving positive results [49]; lysozyme integrated into chitosan nanoparticles showed improved antibacterial activity against E. coli and B. subtilis [50][35]; and a chitosan composite film containing lysozyme–rectorite showed reduced mechanical properties (−28%) in regard to the film containing only chitosan and enhanced antibacterial properties on E. coli and S. aureus [51]. With a different approach on foods already containing egg derivatives, lysozyme immobilized on a polysaccharide was added to the food, in order to improve its functional properties and to extend the shelf life, such as in the case of mayonnaise treated with lysozyme immobilized on Arabic gum [52]. The attempt to reduce the allergenicity of lysozyme-treated wine using a thermal or an enzymatic treatment was carried out without producing a convincing outcome [44].
Since lysozyme exhibited weak inhibitory effects against Gram-negative bacteria, when used alone, the practical application of free lysozyme as a food preservative was quite limited and many methods used to enhance the susceptibility of Gram-negative bacteria were attempted, namely the combined use with cinnamaldehyde [53]; acidic electrolyzed oxidizing water [54]; heat treatment and hydrogen peroxide associated with a packaging in a controlled atmosphere [55]; high hydrostatic pressure [56]; disodium ethylenediaminetetraacetate salt and ethylenediaminetetraacetic acid [57][58]; chitooligosaccharides [59]; chitosan-organic rectorite composites and negatively charged sodium alginate film-coated cellulose acetate mats [60]; EDTA in starch-based active food packaging film [61]; enterocin AS-48 [62]; and pomegranate peel extract [63]. The relevant data on the synergic uses of these substances with lysozyme are summarized in Table 1.
Table 1. Relevant data on the synergic uses of different substances with lysozyme, in order to enhance the susceptibility of Gram-negative bacteria.
| Combined Use with | Application/Claimed Use | Antibiotic Activity Evaluated Against/On | Refs. |
|---|---|---|---|
| Cinnamaldehyde | Storage of olive flounder (Paralichthys olivaceus) fillets/lowered total viable count | S. putrefaciens and P. fluorescens | [53] |
| Acidic electrolyzed oxidizing water | To prolong the shelf life of carp fillets from a microbiological point of view | Total viable count, Enterobacteriaceae count, and anaerobic mesophilic count | [54] |
| Heat treatment and dilution with hydrogen peroxide associated with packaging in a controlled atmosphere | To extend the shelf life of pork meat by more than 20%, compared with the control sample without lysozyme | Aerobic plate count, Enterobacteriaceae, Pseudomonas spp., and lactic acid bacteria | [55] |
| High hydrostatic pressure | Not significant enough to differentiate lethality of lysozyme without additives in cheeses made from raw milk | B. cereus | [56] |
| Disodium ethylenediaminetetraacetate salt | Buffalo meat in refrigerated conditions | Total viable mesophilic count, total viable psychrotrophic count, lactic acid bacteria, Pseudomonas spp., and B. thermosphacta |
[57] |
| Chitooligosaccharides | The microbiological quality of minced meat stored in refrigerated conditions was improved | Escherichia coli, Pseudomonas fluorescens, Bacillus cereus, and Staphylococcus aureus | [59] |
| Ethylenediaminetetraacetic acid disodium salt | To reduce the growth of Y. enterocolitica in orange beverages | Y. enterocolitica | [64] |
| Layer-by-layer chitosan-organic rectorite composites and negative charged sodium alginate. | The sensory analysis and physicochemical analysis applied to assess the effects of layer-by-layer film coating confirmed a higher score for the packaged pork (4 °C for 21 days) | E. coli and S. aureus | [60] |
| EDTA in anti-microbial starch-based active food packaging films | Cooked rice with pulses/effective to inhibit the growth of spoilage microorganisms | Contaminated from the open environmental sources (mainly Gram-negative cocci) | [61] |
| EDTA | A different lysozyme activity against the tested microorganisms was observed, increasing the ratio of lysozyme/EDTA from 11.14:8.14 mg/mL to 11.14:14.14 mg/mL. | Micrococcus lysodeikticus and Escherichia coli | [58] |
| Enterocin AS-48 | Synergy confirmed in liquid whole eggs, egg whites, and egg yolks, at 4 °C and 28 °C | B. cereus and S. aureus | [62] |
| Pomegranate peel extract | To maintain the quality of mackerel fillets wrapped with gelatin/polycaprolactone composite film and to prolong the shelf life of the product | Total mesophilic counts and psychrotrophic bacteria counts | [63] |
References
- Hisham R Ibrahim; Tetsuji Matsuzaki; Takayoshi Aoki; Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Letters 2001, 506, 27-32, 10.1016/S0014-5793(01)02872-1.
- Barbara Masschalck; Rob Van Houdt; Ellen G. R. Van Haver; Chris W. Michiels; Inactivation of Gram-Negative Bacteria by Lysozyme, Denatured Lysozyme, and Lysozyme-Derived Peptides under High Hydrostatic Pressure. Applied and Environmental Microbiology 2001, 67, 339-344, 10.1128/aem.67.1.339-344.2001.
- Isaac Ginsburg; Bactericidal cationic peptides can also function as bacteriolysis-inducing agents mimicking beta-lactam antibiotics?; it is enigmatic why this concept is consistently disregarded. Medical Hypotheses 2004, 62, 367-374, 10.1016/j.mehy.2003.11.017.
- James A. Nash; Tiffany Nicole S. Ballard; Timothy E. Weaver; Henry T. Akinbi; The Peptidoglycan-Degrading Property of Lysozyme Is Not Required for Bactericidal Activity In Vivo. The Journal of Immunology 2006, 177, 519-526, 10.4049/jimmunol.177.1.519.
- Charmaine M. Woods; David N. Hooper; Eng H. Ooi; Lor-Wai Tan; A. Simon Carney; Human Lysozyme has Fungicidal Activity against Nasal Fungi. American Journal of Rhinology & Allergy 2011, 25, 236-240, 10.2500/ajra.2011.25.3631.
- Sylvia Lee-Huang; Paul Huang; Yongtao Sun; Hsiang-Fu Kung; Diana L. Blithe; Hao-Chia Chen; Lysozyme and RNases as anti-HIV components in -core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Sciences 1999, 96, 2678-2681, 10.1073/pnas.96.6.2678.
- A. M. Pendzhiev; Proteolytic Enzymes of Papaya: Medicinal Applications. Pharmaceutical Chemistry Journal 2002, 36, 315-317, 10.1023/a:1020832807958.
- Ikram Khémiri; Badiaa Essghaier; Najla Sadfi-Zouaoui; Lotfi Bitri; Antioxidant and Antimicrobial Potentials of Seed Oil from Carthamus tinctorius L. in the Management of Skin Injuries. Oxidative Medicine and Cellular Longevity 2020, 2020, 1-12, 10.1155/2020/4103418.
- Karachi, A.; Rajaian, H.; Aminlari, M.M.; Tabatabaee, A.; Application of lysozyme and dextran conjugated lysozyme as natural antimicrobial agents in the treatment of experimental skin wound in mice.. Int. J. Pharm. Sci. Res. 2013, 4, 4236 - 4244, 10.13040/IJPSR.0975-8232.4(11).4236-44.
- Christoph Öhlknecht; Gregor Tegl; Bianca Beer; Christoph Sygmund; Roland Ludwig; Georg M. Guebitz; Cellobiose dehydrogenase and chitosan-based lysozyme responsive materials for antimicrobial wound treatment. Biotechnology and Bioengineering 2016, 114, 416-422, 10.1002/bit.26070.
- Adel Abouhmad; Gashaw Mamo; Tarek Dishisha; Magdy Aly Amin; Rajni Hatti‐Kaul; T4 lysozyme fused with cellulose-binding module for antimicrobial cellulosic wound dressing materials. Journal of Applied Microbiology 2016, 121, 115-125, 10.1111/jam.13146.
- Joshua Ravensdale; Fiona Wood; Francis O'brien; Keith Gregg; Investigations into methods to improve the antibacterial activity of Acticoat. Journal of Medical Microbiology 2016, 65, 397-405, 10.1099/jmm.0.000246.
- Mandana Tavakolian; Mira Okshevsky; Theo G. M. Van De Ven; Nathalie Tufenkji; Developing Antibacterial Nanocrystalline Cellulose Using Natural Antibacterial Agents. ACS Applied Materials & Interfaces 2018, 10, 33827-33838, 10.1021/acsami.8b08770.
- Ling Xiao; Wenqiang Ni; Xiaohong Zhao; Yicheng Guo; Xue Li; Fan Wang; Gaoxing Luo; Rixing Zhan; Xisheng Xu; A moisture balanced antibacterial dressing loaded with lysozyme possesses antibacterial activity and promotes wound healing. Soft Matter 2021, 17, 3162-3173, 10.1039/d0sm02245d.
- Ai-Ho Liao; Chi-Ray Hung; Chieh-Fu Lin; Yi-Chun Lin; Hang-Kang Chen; Treatment effects of lysozyme-shelled microbubbles and ultrasound in inflammatory skin disease. Scientific Reports 2017, 7, 41325, 10.1038/srep41325.
- Ruben Cebrian; Sergio Arévalo; Susana Rubiño; Salvador Arias-Santiago; María Dolores Rojo; Manuel Montalbán López; Manuel Martínez Bueno; Eva Valdivia; Mercedes Maqueda; Control of Propionibacterium acnes by natural antimicrobial substances: Role of the bacteriocin AS-48 and lysozyme. Scientific Reports 2018, 8, 1-11, 10.1038/s41598-018-29580-7.
- Di Schiena, M.G.; Ferrari, S.; Rongen, R. Lysozyme Gel Formulations for Use as Disinfectants. U.S. Patent US20130259852, 3 October 2013.
- Haoqi Tan; Dawei Jin; Xue Qu; Huan Liu; Xin Chen; Meng Yin; Changsheng Liu; A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2018, 192, 392-404, 10.1016/j.biomaterials.2018.10.047.
- M. Lourdes Ramiro-Gutiérrez; Julia Will; Aldo R. Boccaccini; Aranzazu Diaz Cuenca; Reticulated bioactive scaffolds with improved textural properties for bone tissue engineering: Nanostructured surfaces and porosity. Journal of Biomedical Materials Research Part A 2013, 102, 2982-2992, 10.1002/jbm.a.34968.
- Victor H. Pino-Ramos; Guadalupe G. Flores-Rojas; Carmen Alvarez-Lorenzo; Angel Concheiro; Emilio Bucio; Graft copolymerization by ionization radiation, characterization, and enzymatic activity of temperature-responsive SR- g -PNVCL loaded with lysozyme. Reactive and Functional Polymers 2018, 126, 74-82, 10.1016/j.reactfunctpolym.2018.03.002.
- Mengqin Yuan; Fangfang Dai; Dan Li; Yaqi Fan; Wei Xiang; Fenghua Tao; Yanxiang Cheng; Hongbing Deng; Lysozyme/collagen multilayers layer-by-layer deposited nanofibers with enhanced biocompatibility and antibacterial activity. Materials Science and Engineering: C 2020, 112, 110868, 10.1016/j.msec.2020.110868.
- Bassam M. Al Meslmani; Gihan F. Mahmoud; Thomas Leichtweiß; Boris Strehlow; Frank O. Sommer; Michael D. Lohoff; Udo Bakowsky; Covalent immobilization of lysozyme onto woven and knitted crimped polyethylene terephthalate grafts to minimize the adhesion of broad spectrum pathogens. Materials Science and Engineering: C 2016, 58, 78-87, 10.1016/j.msec.2015.08.001.
- Takashi Nezu; Tomoyuki Masuyama; Kaori Sasaki; Setsuo Saitoh; Masayuki Taira; Yoshima Araki; Effect of pH and addition of salt on the adsorption behavior of lysozyme on gold, silica, and titania surfaces observed by quartz crystal microbalance with dissipation monitoring.. Dental Materials Journal 2008, 27, 573-580, 10.4012/dmj.27.573.
- Huaqiong Li; Chenyuan Gao; Lin Tang; Chenou Wang; Qiong Chen; QianYi Zheng; Shuoshuo Yang; Sunren Sheng; Xingjie Zan; Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Applied Bio Materials 2019, 3, 673-684, 10.1021/acsabm.9b01017.
- Longwen He; Ye Cui; Chao Zhang; The corrosion resistance, cytotoxicity, and antibacterial properties of lysozyme coatings on orthodontic composite arch wires. RSC Advances 2020, 10, 18131-18137, 10.1039/d0ra02988b.
- Budny, J.A.; Budny, M.J. Compositions for Treating Biofilm. U.S. Patent US6830745, 14 December 2004.
- Frank, K.L.; Rouchon, C.; Harris, J.A. Antibacterial Methods and Related Kits of Treating a Bacterial Infection Using Lysozyme. Patent No. WO2019018368, 24 January 2019.
- Anne Caro; Vincent Humblot; Christophe Méthivier; Michel Minier; Michele Salmain; Claire-Marie Pradier; Grafting of Lysozyme and/or Poly(ethylene glycol) to Prevent Biofilm Growth on Stainless Steel Surfaces. The Journal of Physical Chemistry B 2009, 113, 2101-2109, 10.1021/jp805284s.
- Shaojun Yuan; Jia Yin; Wei Jiang; Bin Liang; S.O. Pehkonen; Cleo Choong; Enhancing antibacterial activity of surface-grafted chitosan with immobilized lysozyme on bioinspired stainless steel substrates. Colloids and Surfaces B: Biointerfaces 2013, 106, 11-21, 10.1016/j.colsurfb.2012.12.048.
- F Devlieghere; A Vermeulen; J Debevere; Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiology 2004, 21, 703-714, 10.1016/j.fm.2004.02.008.
- Roberto Díez-Martínez; Héctor D. De Paz; Héctor David De Paz Fernández; Noemí Bustamante; Chad W. Euler; Vincent A. Fischetti; Margarita Menéndez; Pedro García; A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. Journal of Antimicrobial Chemotherapy 2015, 70, 1763-1773, 10.1093/jac/dkv038.
- Wei Ren; Shujun Wang; Mingsheng Lü; Xiaobei Wang; Yaowei Fang; Yuliang Jiao; Jianen Hu; Optimization of four types of antimicrobial agents to increase the inhibitory ability of marine Arthrobacter oxydans KQ11 dextranase mouthwash. Chinese Journal of Oceanology and Limnology 2015, 34, 354-366, 10.1007/s00343-015-4376-3.
- Ichie Okamoto; Hirofumi Miyaji; Saori Miyata; Kanako Shitomi; Tsutomu Sugaya; Natsumi Ushijima; Tsukasa Akasaka; Satoshi Enya; Satoshi Saita; Hideya Kawasaki; et al. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega 2021, 6, 9279-9290, 10.1021/acsomega.1c00838.
- Xue Zhong; Yunjia Song; Peng Yang; Yao Wang; Shaoyun Jiang; Xu Zhang; Changyi Li; Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLOS ONE 2016, 11, e0146957, 10.1371/journal.pone.0146957.
- Binbin Guan; Haorong Wang; Ruiqing Xu; Guoying Zheng; Jie Yang; Zihao Liu; Man Cao; Mingyao Wu; Jinhua Song; Neng Li; et al.Ting LiQing CaiXiaoping YangYanqiu LiXu Zhang Establishing Antibacterial Multilayer Films on the Surface of Direct Metal Laser Sintered Titanium Primed with Phase-Transited Lysozyme. Scientific Reports 2016, 6, 36408, 10.1038/srep36408.
- Sarra Sebaa; Nicolas Hizette; Zahia Boucherit-Otmani; Philippe Courtois; Dose-dependent effect of lysozyme upon Candida albicans biofilm. Molecular Medicine Reports 2017, 15, 1135-1142, 10.3892/mmr.2017.6148.
- Abdulrahman A. Kehail; Christopher J. Brigham; Anti-biofilm Activity of Solvent-Cast and Electrospun Polyhydroxyalkanoate Membranes Treated with Lysozyme. Journal of Polymers and the Environment 2017, 26, 66-72, 10.1007/s10924-016-0921-1.
- Yilin Hou; Zhaojie Wang; Peng Zhang; Hu Bai; Yuelin Sun; Jinyou Duan; Haibo Mu; Lysozyme Associated Liposomal Gentamicin Inhibits Bacterial Biofilm. International Journal of Molecular Sciences 2017, 18, 784, 10.3390/ijms18040784.
- Mohamed ElAdawy; Mohammed El-Mowafy; Mohamed Mohamed Adel El-Sokkary; Rasha Barwa; Effects of Lysozyme, Proteinase K, and Cephalosporins on Biofilm Formation by Clinical Isolates of Pseudomonas aeruginosa.. Interdisciplinary Perspectives on Infectious Diseases 2020, 2020, 6156720-9, 10.1155/2020/6156720.
- Sharma Komal; Prajapati Abhishek; Shukla Mansi; Gupte Shilpa; Synergistic Effect of Antibiotics and Enzymes as Strategies For Combating Biofilm Formation By Pseudomonas Aeruginosa Pao1. International Journal of pharma and Bio Sciences 2020, 11, 168–178, 10.22376/ijpbs.202011.4.b168-178.
- Olivier Thellin; Willy Zorzi; Lysozyme as a cotreatment during antibiotics use against vaginal infections: An in vitro study on Gardnerella vaginalis biofilm models. null 2016, 19, 101-107, 10.2436/20.1501.01.268.
- Anirudh Singh; Arushi Verma; Ruhar Singh; Amaresh Kumar Sahoo; Sintu Kumar Samanta; Combination therapy of biogenic C-dots and lysozyme for enhanced antibacterial and antibiofilm activity. Nanotechnology 2020, 32, 085104, 10.1088/1361-6528/abc2ed.
- P. D'Incecco; M. Gatti; Johannes Hogenboom; Benedetta Bottari; V. Rosi; E. Neviani; Luisa Maria Pellegrino; Lysozyme affects the microbial catabolism of free arginine in raw-milk hard cheeses. Food Microbiology 2016, 57, 16-22, 10.1016/j.fm.2015.11.020.
- Wilman Carrillo; A. Garcia-Ruiz; I. Recio; M. V. Moreno-Arribas; Antibacterial Activity of Hen Egg White Lysozyme Modified by Heat and Enzymatic Treatments against Oenological Lactic Acid Bacteria and Acetic Acid Bacteria. Journal of Food Protection 2014, 77, 1732-1739, 10.4315/0362-028x.jfp-14-009.
- van Landschoot, A.; Villa, A.; Antibacterial properties of hen egg white lysozyme against beer spoilage bacteria and effect of lysozyme on yeast fermentation. Cerevisia 2008, 32, 219-224.
- Qiuying Li; Jinxiu Xu; Dongdong Zhang; Keli Zhong; Tong Sun; Xuepeng Li; Jianrong Li; Preparation of a bilayer edible film incorporated with lysozyme and its effect on fish spoilage bacteria. Journal of Food Safety 2020, 40, e12832, 10.1111/jfs.12832.
- S.-I. Park; M.A. Daeschel; Y. Zhao; Functional Properties of Antimicrobial Lysozyme-Chitosan Composite Films. Journal of Food Science 2004, 69, M215-M221, 10.1111/j.1365-2621.2004.tb09890.x.
- S. Amiri; R. Ramezani; M. Aminlari; Antibacterial Activity of Dextran-Conjugated Lysozyme against Escherichia coli and Staphylococcus aureus in Cheese Curd. Journal of Food Protection 2008, 71, 411-415, 10.4315/0362-028x-71.2.411.
- Chedia Ben Amara; Noushin Eghbal; Nadia Oulahal; Pascal Degraeve; Adem Gharsallaoui; Properties of lysozyme/sodium alginate complexes for the development of antimicrobial films. Food Research International 2016, 89, 272-280, 10.1016/j.foodres.2016.08.015.
- Tiantian Wu; Chunhua Wu; Shalu Fu; Liping Wang; Chunhong Yuan; Shiguo Chen; Yaqin Hu; Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydrate Polymers 2017, 155, 192-200, 10.1016/j.carbpol.2016.08.076.
- Xiang Li; Hu Tu; Mengtian Huang; Jiajia Chen; Xiaowen Shi; Hongbing Deng; Shuangfei Wang; Yumin Du; Incorporation of lysozyme-rectorite composites into chitosan films for antibacterial properties enhancement. International Journal of Biological Macromolecules 2017, 102, 789-795, 10.1016/j.ijbiomac.2017.04.076.
- Marjan Mohammadi Hashemi; Mahmoud Aminlari; Mehdi M. Forouzan; Esmaeel Moghimi; Maryam Tavana; Shahram Shekarforoush; Mohammad Amin Mohammadifar; Seyed Shahram Shekarforoush; Production and Application of Lysozyme-Gum Arabic Conjugate in Mayonnaise as a Natural Preservative and Emulsifier. Polish Journal of Food and Nutrition Sciences 2018, 68, 33-43, 10.1515/pjfns-2017-0011.
- Yongxia Xu; Yiming Yin; Tao Li; Honglei Zhao; Xuepeng Li; Jianrong Li; Tong Sun; Effects of lysozyme combined with cinnamaldehyde on storage quality of olive flounder (Paralichthys olivaceus) fillets. Journal of Food Science 2020, 85, 1037-1044, 10.1111/1750-3841.14980.
- Péter Palotás; Gábor Jónás; József Lehel; László Friedrich; Preservative Effect of Novel Combined Treatment with Electrolyzed Active Water and Lysozyme Enzyme to Increase the Storage Life of Vacuum-Packaged Carp. Journal of Food Quality 2020, 2020, 1-7, 10.1155/2020/4861471.
- Renata Cegielska-Radziejewska; Tomasz Szablewski; Elżbieta Radziejewska-Kubzdela; Łukasz Tomczyk; Agata Biadała; Grzegorz Leśnierowski; The Effect of Modified Lysozyme Treatment on the Microflora, Physicochemical and Sensory Characteristics of Pork Packaged in Preservative Gas Atmospheres. Coatings 2021, 11, 488, 10.3390/coatings11050488.
- Tomás J. López-Pedemonte; Artur Xavier Roig Sagués; Antonio-José Trujillo; Marta Capellas; Buenaventura Guamis; Inactivation of Spores of Bacillus cereus in Cheese by High Hydrostatic Pressure with the Addition of Nisin or Lysozyme. Journal of Dairy Science 2003, 86, 3075-3081, 10.3168/jds.s0022-0302(03)73907-1.
- Marianna Cannarsi; Antonietta Baiano; Milena Sinigaglia; Lino Ferrara; Rodolfo Baculo; Matteo A. Del Nobile; Use of nisin, lysozyme and EDTA for inhibiting microbial growth in chilled buffalo meat. International Journal of Food Science & Technology 2008, 43, 573-578, 10.1111/j.1365-2621.2006.01438.x.
- Mulia W. Apriliyani; Djalal Rosyidi; Purwadi Purwadi; Hari Purnomo; Abdul Manab; The Release of Egg White Lysozyme Containing EDTA from Composite Edible Film Based on Whey Protein, Konjac Flour and Lipid. Advance Journal of Food Science and Technology 2014, 6, 48-55, 10.19026/ajfst.6.3029.
- M.S. Rao; R. Chander; A. Sharma; Synergistic effect of chitooligosaccharides and lysozyme for meat preservation. LWT 2008, 41, 1995-2001, 10.1016/j.lwt.2008.01.013.
- Weijuan Huang; Huijinlan Xu; Yue Xue; Rong Huang; Hongbing Deng; Siyi Pan; Layer-by-layer immobilization of lysozyme–chitosan–organic rectorite composites on electrospun nanofibrous mats for pork preservation. Food Research International 2012, 48, 784-791, 10.1016/j.foodres.2012.06.026.
- Sugandha Bhatia; Anoop Bharti; Evaluating the antimicrobial activity of Nisin, Lysozyme and Ethylenediaminetetraacetate incorporated in starch based active food packaging film. Journal of Food Science and Technology 2014, 52, 1-9, 10.1007/s13197-014-1414-7.
- Samir Ananou; Silvia Rivera; Maria Isabel Madrid; Mercedes Maqueda; Manuel Martínez-Bueno; Eva Valdivia; Application of enterocin AS-48 as biopreservative in eggs and egg fractions: Synergism through lysozyme. LWT 2018, 89, 409-417, 10.1016/j.lwt.2017.11.018.
- Ainaz Khodanazary; Quality characteristics of refrigerated mackerel Scomberomorus commerson using gelatin-polycaprolactone composite film incorporated with lysozyme and pomegranate peel extract. International Journal of Food Properties 2019, 22, 2057-2071, 10.1080/10942912.2019.1702997.
- Cecilia S.M. Lucero Estrada; Lidia Del Carmen Velázquez; Ana María S. De Guzman; EFFECTS OF ORGANIC ACIDS, NISIN, LYZOZYME AND EDTA ON THE SURVIVAL OFYERSINIA ENTEROCOLITICAPOPULATION IN INOCULATED ORANGE BEVERAGES. Journal of Food Safety 2010, 30, 24-39, 10.1111/j.1745-4565.2009.00187.x.
- Cecilia S.M. Lucero Estrada; Lidia Del Carmen Velázquez; Ana María S. De Guzman; EFFECTS OF ORGANIC ACIDS, NISIN, LYZOZYME AND EDTA ON THE SURVIVAL OFYERSINIA ENTEROCOLITICAPOPULATION IN INOCULATED ORANGE BEVERAGES. Journal of Food Safety 2010, 30, 24-39, 10.1111/j.1745-4565.2009.00187.x.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.6K
Revisions:
2 times
(View History)
Update Date:
24 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




