Allium cepa (onion) is one of the main
Allium spices that were studied for their beneficial effects on human health, specifically on the cardiovascular system. Their antioxidant, antihypertensive, antiplatelet, anti-inflammatory and antihyperlipidemic effects were analysed in various settings
[1][2]. Onions are a widely cultivated and consumed vegetable all over the world, although they originate from Central Asia. Traditionally, onions were used to treat cold, flu, dysentery, wound healing, and alleviate pain
[2]. Several studies have explored the different classes of phytochemicals present in
A. cepa including volatile oil, and sulphur-containing compounds such as methyl 5-methylfuryl sulphide and dimethyl disulphide which are responsible for their characteristic flavour. In addition, phenolic compounds such as phenolic acids (e.g.,
p-hydroxybenzoic acid and gallic acid) and flavonoids (e.g., anthocyanins, quercetin and kaempferol) were isolated and characterised from onions
[3][4][5]. These active constituents are associated with the biological effects of
A. cepa, including antioxidant, antibiotic, anti-cancer, anti-diabetic, anti-inflammatory and anti-allergic activities. In addition, they significantly reduce CVD risks through hypolipidemic, hypotensive, hypoglycaemic and antiplatelet effects
[1][2].
To evaluate the antiplatelet effects of
A. cepa bulb, different concentrations of its aqueous extracts (250 and 500 mg/mL) were tested in human isolated platelets using an aggregation assay upon stimulation with a TXA
2 receptor agonist, U46619 (2 µM). Both concentrations of the extract (250 and 500 mg/mL) significantly inhibited platelet aggregation by around 85–100%
[6]. In addition, an ethanolic extract of
A. cepa bulb showed significant antiplatelet effects when 5 µg/mL collagen was used as an agonist in rat isolated platelets through reducing intracellular Ca
2+ levels, cyclooxygenase 1 enzyme (COX-1) and TXA
2 synthase activities. It also increased cAMP levels in a concentration dependent manner
[7]. The anti-aggregatory effects of
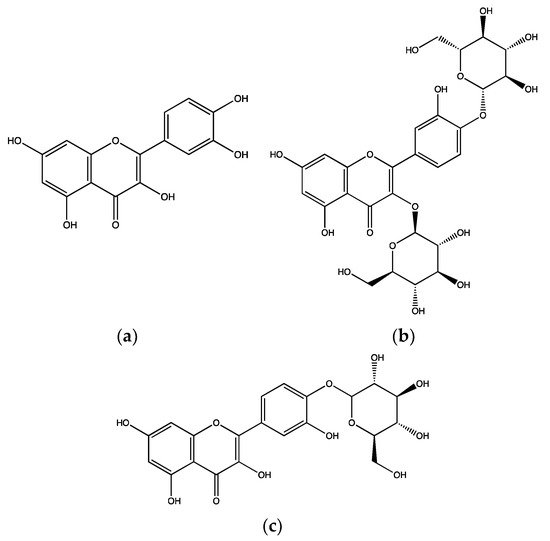
A. cepa are attributable to the abundant flavonoid, quercetin (
Figure 1a) and its glycosides, quercetin-3,4′-
O-diglucoside (
Figure 1b) and quercetin-4′-
O-monoglucoside (
Figure 1c). These compounds were isolated from the methanolic extract of
A. cepa and at a concentration of 2 mg/mL, they completely inhibited 6 µg/mL collagen-induced platelet (rat) aggregation
[8].
Figure 1. Chemical structures of quercetin (a), quercetin-3,4′-O-diglucoside (b) and quercetin-4′-O-monoglucoside (c) isolated from Allium cepa.
Furthermore, to test the impact of cooking methods and cooking time on
A. cepa bulb-mediated antiplatelet effects, conventional (200 °C) and microwave (500 W, which is almost equivalent to 200 °C) ovens were selected to cook the samples for 10, 20 or 30 min. For conventional oven cooking,
A. cepa samples were divided into; whole (intact) bulb, chopped into quarters, and crushed samples. For microwave cooking, only the whole bulbs and crushed samples were tested. Then, all samples were tested in human whole blood aggregation upon stimulation with 1 µg/mL collagen. First, a raw crushed sample of
A. cepa was tested and it significantly inhibited platelet aggregation by around 85%. Samples that were cooked using the conventional oven showed different effects on platelet aggregation compared to the raw samples as shown in
Table 1. These data suggested that the antiplatelet effects of
A. cepa bulb can be lost due to aggressive processing using high temperature. In addition, the long cooking time can change the anti-aggregatory effects of
A. cepa. On the other hand, samples that were cooked using the microwave method did not exert any inhibitory effects on platelets, irrespective of the cooking time
[9].
Table 1. The effects of conventional oven and microwave cooking, different preparation methods and cooking time on antiplatelet activities of
A. cepa [9].
| Cooking Time (min). |
Type of Processing |
Effects on Platelet Aggregation |
| Conventional Oven Cooking |
| 10 |
Crushed |
No inhibition or activation effects |
| 20 & 30 |
Pro-aggregatory by ~25% |
| 10 |
Chopped into quarters |
85% inhibition of aggregation |
| 20 & 30 |
Pro-aggregatory by ~40% |
| 10 & 20 |
Whole (intact) bulb |
85% inhibition of aggregation |
| 30 |
Pro-aggregatory by ~30% |
| Microwave cooking |
| 2 |
Crushed |
Insignificant inhibition |
| 4 |
No inhibition or activation effects |
| 8 |
Pro-aggregatory by ~25% |
| 2 |
Whole (intact) bulb |
Pro-aggregatory by ~20% |
| 4 |
Pro-aggregatory by ~30% |
| 8 |
Pro-aggregatory by ~40% |
Furthermore, a human pilot study (
n = 6) tested the acute effects of low (8.1 mg/L) and high (114.8 mg/L) quercetin (
Figure 1a) contents in onion soups on human platelet activity via oral consumption. The anti-aggregatory effects of onion soups were evaluated in human isolated platelets upon activation with collagen (0.5, 1, 2 and 3 µg/mL) after 1 and 3 h of consumption. The soup with high quercetin content significantly inhibited platelet aggregation induced by different concentrations of collagen. In addition, the effect of both soups on tyrosine phosphorylation of spleen tyrosine kinase (Syk) and phospholipase C gamma 2 (PLCγ2) were evaluated, as they are crucial molecules in the signalling pathways of GPVI (a major collagen receptor). The high quercetin soup significantly inhibited the phosphorylation of Syk and PLCγ2 in 25 µg/mL collagen-induced platelets, when samples collected after 1 and 3 h of ingestion. In addition, the low quercetin soup insignificantly inhibited platelet aggregation induced by collagen while it stimulated tyrosine phosphorylation of Syk and PLCγ2 compared to the control at 1 and 3 h after ingestion (
Figure 2)
[10].
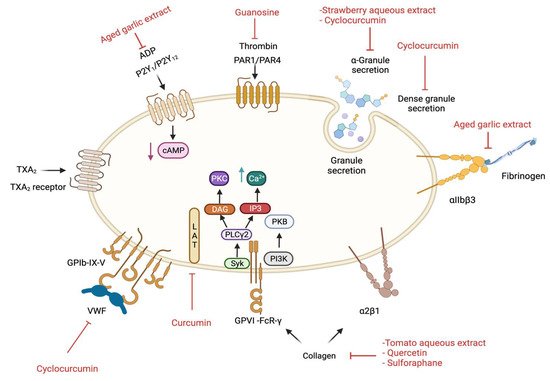
Figure 2. Signalling pathways that are affected by selective plant extracts and their compounds in platelets. cAMP; cyclic adenosine monophosphate, DAG; diacylglycerol, IP3; inositol trisphosphate, PI3K; phosphoinositide 3-kinase, PKB; Protein kinase B, PKC; Protein kinase C, PLCγ2; phospholipase Cγ2, and Syk; spleen tyrosine kinase. This image was created in
BioRender.com (accessed on 23 December 2021). using the information provided in this article.
2. Garlic
Allium sativum (garlic) is a common used vegetable and plays a critical role in the traditional medicine of many ancient cultures, such as the Egyptian, Indian, Chinese, Sumerian, and Greek. It was widely used to treat persistent cough, arthritis, constipation, snakebites and as a general antibiotic
[11]. Various studies demonstrated that
A. sativum exerts antioxidant, anti-inflammatory, anti-tumor, antibiotic, hypoglycaemic and renal protective effects
[11][12]. In addition, it was reported that
A. sativum has positive effects on CVDs through its antioxidant, hypotensive and hypocholesterolaemic effects. These biological effects are linked to sulphur-containing phytochemicals (including alliin and allicin) and enzymes (including alliinase and peroxidase) as well as flavonoids (e.g., quercetin) in
A. sativum [12]. The antiplatelet effects of aqueous and methanolic extracts of
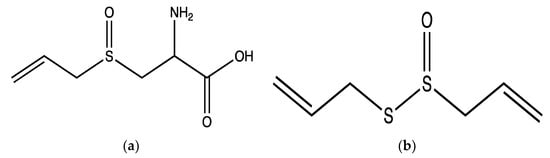
A. sativum bulbs were examined in human PRP aggregation using different agonists; 20 µM ADP, 190 µg/mL collagen and 20 µM epinephrine. The aqueous extracts (10 mg/mL) significantly reduced ADP-induced aggregation by around 86% but did not affect aggregation induced by other agonists. However, the methanolic extracts (10 mg/mL) significantly inhibited ADP, epinephrin and collagen-induced aggregation by approximately 89%, 66% and 32%, respectively. The antiaggregatory effects of the methanolic extracts were suggested to be as a result of high contents of alliin (
Figure 3a) and allicin (
Figure 3b) in this plant
[13]. However, the tested concentrations of agonists were higher than the commonly used concentrations (ADP: 0.5–10 µM; collagen: 1–5 µg/mL; epinephrin: 0.5–10 µM) for platelet aggregation
[13]. Therefore, the inhibitory effects were not apparent.
Figure 3. Structure of alliin (a) and (b) allicin isolated from the methanolic extract of A. sativum.
Allicin is an organosulfur compound that accounts for around 70% of total thiosulfinates [contain the functional group, R-S(O)-S-R] in
A. sativum. It is produced upon the physical disruption (e.g., by crushing or cutting) of tissues of
A. sativum as the alliinase enzyme converts alliin to allicin upon damage
[13]. Allicin and alliin are the main compounds responsible for the biological activities of
A. sativum [12][14][15]. In human PRP and isolated platelets, the effect of 40 µM allicin was tested using 5 µg/mL collagen or 10 µM ADP and 55 µM epinephrine (combined)-induced platelet aggregation. In PRP, allicin did not affect aggregation, whilst in isolated platelets allicin markedly inhibited aggregation by around 98% in collagen and ADP-epinephrine activated platelets which indicates that the anti-aggregatory effects of allicin may be affected by plasma proteins in PRP. In addition, 40 µM allicin demonstrated significant inhibitory effects on fibrinogen binding and P-selectin exposure by around 80% and 90%, respectively, in ADP-epinephrine activated platelets
[15]. However, in this study the combined usage of ADP-epinephrine as agonists was not justified. Moreover, the epinephrine concentration that was used appears to be higher than the commonly used concentrations (0.5–10 µM).
In addition,
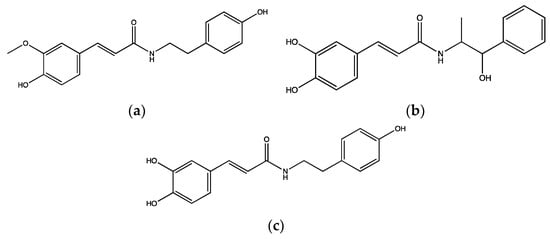
N-feruloyltyramine (
Figure 4a) is an amide alkaloid isolated from methanolic extracts of
A. sativum and it is known to exhibit antioxidant, anti-fungal, anti-bacterial and cytotoxic effects
[16]. This compound was tested in mouse whole blood along with its synthetic analogues,
N-caffeoylnorephedrine (
Figure 4b) and
N-caffeoyltyramine (
Figure 4c) to evaluate their effects on platelet function, specifically on COX-I enzyme and P-selectin exposure.
N-feruloyltyramine and its analogues at the concentration of 0.05 µM significantly reduced the activity of COX-1. However,
N-feruloyltyramine exhibited the highest inhibitory effect of around 43% compared to a COX-1 inhibitor, ibuprofen. In addition,
N-feruloyltyramine,
N-caffeoyltyramine and
N-caffeoylnorephedrine (0.05 µM) significantly inhibited P-selectin exposure by 31%, 30% and 39%, respectively, in mouse whole blood upon stimulation with 2.5 µg/mL collagen
[17].
Figure 4. Chemical structures of (a) N-feruloyltyramine, (b) N-caffeoylnorephedrine and (c) N-caffeoyltyramine isolated from methanolic extracts of A. sativum.
Moreover, the extracts of
A. sativum were evaluated after 20 months following their storage (aged extracts) for their antiplatelet effects. The aged extract was prepared by placing the chopped
A. sativum bulb in ethanol or water/ethanol mixture at room temperature for 20 months. During this process, allicin, which is an unstable compound, is converted to a stable compound, namely
S-allylcysteine. In addition, this extract has a higher content of total phenolic compounds compared to fresh
A. sativum extracts (around 129 ± 1.8 mg/g compared to 56 ± 1.2 mg/g). Aged extracts showed better antioxidant, hypotensive, hypoglycaemic and hypolipidemic effects than fresh
A. sativum extracts
[18]. The aged extract prepared in 15–20% water: ethanol was tested in 8 µM ADP-induced human PRP aggregation at different concentrations; 0.29%, 0.58%, 1.56%, 3.12% and 6.25% (
v/v). All these concentrations significantly inhibited platelet aggregation and fibrinogen binding (
Figure 2) in a concentration dependant manner. Additionally, the extract markedly affected the change of platelet shape by blocking filopodia formation upon stimulation with ADP
[19]. In another study, an aged extract [1.56%, 3.12%, 6.25%,12.5% and 25% (
v/v)] significantly reduced human PRP aggregation induced by 8 µM ADP in a concentration dependant manner with almost 75% inhibition achieved at 25% (
v/v) of the extract. In addition, 25% (
v/v) extract significantly suppressed intracellular Ca
2+ levels in 5 µM A23187 (a calcium ionophore) activated human isolated platelets
[20]. Moreover, at concentrations of 3.12% and 12.5% (
v/v), it markedly inhibited ADP-induced human PRP aggregation by around 40% by reducing the binding of platelets to fibrinogen and increasing the levels of intracellular cAMP (
Figure 2)
[21].
Furthermore, the antiplatelet effects of the aged extract were tested in rat PRP following oral administration. Three doses (1, 2 or 5 g/kg/day) of the extract were administered for 7 or 14 days in different cohorts of rats and then PRP was tested using 10 µg/mL collagen. All doses that were administered for 14 days significantly inhibited aggregation in a dose dependent manner. In isolated platelets obtained from rats treated with a 5 g/kg/day dose for 14 days, a significant inhibition of the phosphorylation of the mitogen-activated protein (MAP) kinases; p38, c-JUN NH2-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) (following activation with collagen), which are all important for platelets signalling was observed
[22].
3. Wild Garlic
Allium ursinum has a less pungent taste than
A. sativum (common garlic) and all of its parts are edible, although the leaves are typically consumed (raw or cooked) rather than the cloves, due to their high content of biologically active compounds, specifically organosulfur molecules
[23]. The leaves were used in Asian, Middle Eastern and European folk medicine for their anti-bacterial, digestion stimulating and hypotensive effects
[24]. Recent studies have demonstrated its antibiotic, cytotoxic, hypotensive, hypolipidemic and antiplatelet effects
[23].
A study tested the antiplatelet effects of aqueous, chloroform and methanolic extracts of leaves of
A. ursinum as well as the ethyl acetate fractions of the ethanolic extract (prepared by liquid-liquid extraction) in human PRP upon activation with different agonists. The ethanolic extract (10 mg/mL) showed a significant reduction in 20 µM ADP- induced aggregation by around 66%. However, the inhibitory effects against A23178 (4 µg/mL) and epinephrine (20 µM) were not significant. In addition, chloroform and ethyl acetate fractions of the methanolic extract, at a concentration of 5 mg/mL, inhibited ADP-induced platelet aggregation by almost 98%
[13]. Similarly, the ethanolic extract of
A. ursinum leaves, essential oil, ethyl acetate and chloroform factions showed significant inhibitory effects, specifically against 20 µM ADP-induced human PRP aggregation. In addition, isolated compounds from chloroform fractions such as 1,2-di-
O-α-linolenoyl-3-
O-β-
d-galactopyranosyl-sn-glycerol (
Figure 5a) and β-sitosterol-3-
O-β-
d-glucoside (
Figure 5b) showed inhibitory effects on ADP-induced human PRP aggregation
[24].
Figure 5. Chemical structures of (a) 1,2-di-O-α-linolenoyl-3-O-β-d-galactopyranosyl-sn-glycerol and (b) β-sitosterol-3-O-β-d-glucoside isolated from chloroform fractions of methonolic extract of A. ursinum.
4. Cruciferous Vegetables
Cruciferous vegetables (family Brassicaceae) such as cabbage, Chinese cabbage, cauliflower, broccoli and kale are cultivated and consumed globally
[25]. Various studies have reported on the protective effects of cruciferous vegetables against different types of cancers including lung, breast, gastric, bladder and prostate cancers
[25][26][27][28]. Cruciferous vegetables are a good source of vitamins (including vitamin C, E and folic acid), minerals (including calcium, iron and zinc), flavonoids (mainly anthocyanins), phenolic acids (including hydroxycinnamic acid) and tannins
[25]. However, most of the biological effects of cruciferous vegetables result from their organosulfur compounds (glucosinolates) that are hydrolysed into isothiocyanates and indole-3-carbinol by myrosinase present in plant cells during the process of chopping, cooking or freezing, or in human gut
[25][29][30]. In addition, cruciferous vegetables significantly reduce CVDs-associated mortalities and exhibit antioxidant, hypotensive, hypolipidemic, hypoglycaemic and antiplatelet effects
[30][31].
The ethyl acetate and
n-butanol extracts of
Brassica oleracea L. var.
capitata (cabbage) leaves,
Brassica oleracea var.
Italica (broccoli) florets,
Brassica oleracea var.
botrytis L. (cauliflower) floral head,
Brassica rapa subsp.
rapa (turnip) root and
Wasabia japonica rhizome (wasabi) were evaluated for their antiplatelet effects in human PRP using 10 µM ADP and 0.5 mM arachidonic acid (AA) as agonists (
Table 2). In AA-induced PRP aggregation, the ethyl acetate extracts of
Wasabia japonica,
Brassica oleracea L. var.
capitata and
Brassica rapa subsp.
rapa showed significant inhibitory effects by around 90%, 88% and 80%, respectively. The ethyl acetate extract of
Wasabia japonica inhibited platelets by 60%, while the
n-butanol extract inhibited platelets by 58% upon stimulation with ADP in platelets
[32].
Table 2. Effects of extracts of selected cruciferous vegetables on human-platelet activation induced by ADP and AA
[32].
| |
(%) Inhibition of Platelet Aggregation Induced by |
| Vegetable Name |
Extract |
ADP |
AA |
| Brassica oleracea L. var. capitata (cabbage) |
Ethyl acetate |
28% |
88% |
| Brassica oleracea var. Italica (broccoli) |
40% |
17% |
| Brassica oleracea var. botrytis L. (cauliflower) |
8% |
10% |
| Brassica rapa subsp. rapa (turnip) |
30% |
80% |
| Wasabia japonica (wasabi) |
62% |
90% |
| Brassica oleracea L. var. capitata (cabbage) |
n-Butanol |
22% |
60% |
| Brassica oleracea var. Italica (broccoli) |
33% |
0% |
| Brassica oleracea var. botrytis L. (cauliflower) |
10% |
0% |
| Brassica rapa subsp. rapa (turnip) |
4% |
5% |
| Wasabia japonica (wasabi) |
58% |
62% |
In addition, methanolic extracts of
Brassica oleraceae L. var.
acephala (kale) leaves exhibited significant reduction in P-selectin exposure in AA (250 µg/mL)-induced whole blood samples that were collected from patients who were diagnosed with pathological conditions such as obesity, hypertension, hyperglycaemia, and hyperlipidaemia. However, its effect on agonist-induced fibrinogen binding was insignificant
[33].
The antiplatelet effects of the anthocyanin-rich extract were evaluated in human platelets. Anthocyanins are a group of plant pigments that belong to the flavonoid class of phytochemicals. Their basic structures consist of a flavylium cation (
Figure 6) and based on the substitutions at C3, C5–C7and C3′–C5′ positions, their structures are different in diverse anthocyanins
[34]. Various studies reported the health benefits of anthocyanins including antioxidant, anti-inflammatory, anti-cancer, anti-bacterial, anti-thrombotic and neuroprotective effects
[34][35].
Brassica oleracea var.
capitata F.
rubra (red cabbage) leaves, which are abundant in anthocyanins (around 322 mg of anthocyanins/100 g of fresh weight), were prepared as a methanolic extract. The anthocyanin-rich extract at 5, 10 and 15 µM (calculated based on absorption coefficient) showed a significant inhibition on human isolated platelet aggregation induced by 0.5 U/mL thrombin to around 66%, 53% and 38%, respectively
[36]. This extract also exhibited an inhibitory effect on lipid peroxidation in platelets which leads to inappropriate platelet activation
[36][37]. At a concentration of 15 µM, it significantly reduced the production of superoxide anion in human isolated platelets activated by thrombin (6 U/mL) by around 80%, while at the concentration of 10 µM, it significantly inhibited the metabolism of AA and subsequently the formation of TXA
2 [35][36]. At 10 µM, it significantly suppressed lipid peroxidation in platelets activated by lipopolysaccharides (LPS) from
Escherichia coli and
Pseudomonas aeruginosa (0.15 and 1.5 µg/mL) by around 50% (with
E. coli LPS) and 60% (
P. aeruginosa LPS)
[37].
Figure 6. Basic structure of anthocyanins (flavylium cation).
Sulforaphane (
Figure 7) is a sulphur compound that is abundant in
Brassica oleracea var.
Italica (broccoli) florets (62.64–982.36 µg/g of dry weight) and stem (18.11 to 274.00 µg/g of dry weight)
[38] and in
Brassica oleracea var.
capitata F.
rubra (red cabbage) leaves (48–101.99 µg/g of dry weight) and
Brassica oleracea L. var.
capitata (green cabbage) leaves (7.58–540 µg/g of dry weight)
[39][40]. It has been reported that sulforaphane exhibits anti-cancer, antibiotic, antioxidant, anti-inflammatory, hypotensive and hypoglycaemic effects
[29].
Figure 7. Structure of sulforaphane, an abundant sulphur compound in cruciferous vegetables.
The effect of sulforaphane on platelet aggregation has been evaluated in previous studies
[41][42]. In human isolated platelets, 25 µM and 50 µM sulforaphane markedly inhibited 1 µg/mL collagen-induced platelet aggregation by around 60% and 90%, respectively. However, the inhibitory effects of sulforaphane (25–200 µM) in platelets induced by 60 µM AA, 1 µM U46619 or 0.05 U/mL thrombin were not significantly different from controls. Moreover, 25 µM and 50 µM sulforaphane markedly inhibited the phosphorylation of PLCγ2 in platelets (
Figure 2)
[41]. Upon platelet activation, PLCγ2 hydrolyses membrane phospholipid, phosphatidylinositol 4,5 bisphosphate (PIP
2) to produce the second messengers 1,4,5-trisphosphate (IP
3), which increases the cytosolic calcium levels and diacylglycerol (DAG) to stimulate protein kinase C (PKC) which in turn activates platelet degranulation, TXA
2 synthesis and release, and activation of integrin αIIbβ3 leading to platelet aggregation
[43]. Furthermore, sulforaphane at 0.125 and 0.25 mg/kg showed a significant reduction in mortality rate due to ADP (700 mg/kg) induced acute pulmonary thrombosis in mice by 58.3% and 41.7%, respectively
[41]. Similarly, the effect of 60 µM and 100 µM of sulforaphane on platelet aggregation was tested in human isolated platelets after 30 min of incubation. Upon activation with thrombin (0.1 U/mL), 100 µM sulforaphane showed 50% inhibition. However, in collagen (2.5 µg/mL)-activated platelets, both 60 µM and 100 µM of sulforaphane inhibited aggregation by nearly 100%. However, neither concentrations affected ADP (1.56 µM)-activated platelets
[44]. In addition, aggregation using mouse platelets activated by 3 U/mL thrombin was significantly suppressed by sulforaphane at 10, 20 and 50 µM. In vivo tail bleeding times in mice were also prolonged to 58.2 ± 1.4 and 76.4 ± 1.2 s when 7.1 µg/mouse and 17.7 µg/mouse concentrations were used, respectively
[42].
Another anti-cancer compound isolated from cruciferous vegetables is indole-3-carbinol (
Figure 8). This compound is produced by the hydrolysis of glucobrassicin (which is a glucosinolate) via myrosinase, during plant tissue processing, i.e., chopping, crushing, or chewing or by the human gut microflora. Glucobrassicin is a major compound in
Brassica oleracea var.
Italica (broccoli),
Brassica oleraceae var.
capitata F. alba (white cabbage) and
Brassica oleracea var.
botrytis L. (cauliflower) as it accounts for almost 50% of the total glucosinolate in those vegetables
[45]. Indole-3-carbinol has been reported to possess anticancer effects, specifically against hormone-dependent cancers such as breast, uterine, ovarian and prostate
[46][47].
Figure 8. Structure of indole-3-carbinol isolated from different Brassica species.
Additionally, the antiplatelet effects of indole-3-carbinol were evaluated using various platelet functional assays. Different concentrations of indole-3-carbinol (3, 6, 12 and 25 µM) showed a significant reduction in human isolated platelet aggregation activated by 5 µg/mL collagen by around 70%. Thromboxane B
2 (TXB
2, a stable metabolite of TXA
2) production was also significantly inhibited by 25 µM indole-3-carbinol by around 70%. Moreover, in a thromboembolism model in mice, the oral administration of 4.4, 8.8, 17.7 or 36.8 mg/kg of indole-3-carbinol protected mice against mortality by 67%, 70%, 70% and 89%, respectively upon stimulation with a mixture of 114 µg collagen and 13.20 µg epinephrine
[48].
In another study, indole-3-carbinol (3, 6, 12 and 25 µM) displayed significant inhibitory effects on 10 µM ADP-induced human PRP aggregation by 64%, 76%, 84% and 90%, respectively. Additionally, in ADP (10 µM) activated rat PRP, oral doses of 12.5, 25, and 50 mg/kg/day of indole-3-carbinol for 14 days significantly suppressed PRP activation by 33%, 55%, 66% and 77%, respectively. The same doses significantly reduced rat brain infarction volume in a middle-cerebral artery occlusion rat model
[49].
5. Green Leafy Vegetables
Green leafy vegetables are rich in flavonoids, vitamins such as vitamin A and C, glucosinolates, carotenoids, essential polyunsaturated fatty acids and nitrate
[50][51]. Nitrates (NO
3−) that consist of nitrogen and oxygen atoms are crucial for plant growth as they are used to synthesise amino acids and subsequently proteins. They are also important for chlorophyll formation. Plants absorb nitrates from soil through their roots and then store them in leaves
[52]. Therefore, green leafy vegetables have a high content of nitrates. Nitrates display different pharmacological effects, including antioxidant, gastroprotective, antibacterial, hypotensive, and antiplatelet effects and have been found to improve endothelial function and blood flow to ischaemic tissues
[52][53].
Spinacia oleracea (spinach) leaves have many beneficial effects on human health including antioxidant, anti-cancer, anti-inflammatory, hypolipidemic and hypoglycaemic effects
[54]. Cho et al.
[55][56], studied the effect of
Spinacia oleracea (spinach) leaf extract which is a saponin-rich extract on platelet activity. Saponins are natural compounds that have shown positive impacts on cardiovascular health by acting as antioxidant, hypotensive, hypoglycaemic and hypocholesterolaemic agents
[57]. Different concentrations (100, 300 and 500 µg/mL) of the
S. oleracea saponin-rich extract showed significant inhibitory effects on aggregation in rat isolated platelets upon activation by 10 µg/mL collagen in a concentration dependent manner (53%, 50% and 40%, respectively). In addition, 500 µg/mL saponins-rich extract significantly increased cAMP and cGMP levels in platelets by around 60% compared to the controls
[56].
Another leafy vegetable that demonstrated antiplatelet effects is
Eruca sativa (Rocket) leaves
[58]. 1 mg/mL concentration of 30% methanolic extract of
E. sativa leaves was tested in human PRP, activated by 8 µM ADP, 1 mM AA and 1.5 µg/mL collagen. However, only the ADP-stimulated platelet aggregation was inhibited by around 50% (IC
50 of 0.71 mg/mL). In addition, it inhibited P-selectin exposure from around 58% to 42% and the release of TXB
2 in platelets. A single dose of 200 mg/kg of the extract was tested in a thrombosis model in mice and it postponed the artery occlusion time to 60 min compared to 30 min in the control group and it significantly reduced the maximum occlusion from 100% to almost 56%
[58].
Overall, it is evident that selected fruits, vegetables and spices have beneficial effects on the modulation of platelet activation. Therefore, their regular consumption as part of our diet will be highly beneficial in the prevention and management of CVDs, specifically, thrombotic diseases.