Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takao Yamasaki | + 2282 word(s) | 2282 | 2021-12-28 04:58:52 | | | |
| 2 | Amina Yu | + 662 word(s) | 2944 | 2022-01-19 02:46:55 | | | | |
| 3 | Takao Yamasaki | Meta information modification | 2944 | 2022-07-21 14:08:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yamasaki, T. Subtle Daily Behavioral Changes and Mild Cognitive Impairment. Encyclopedia. Available online: https://encyclopedia.pub/entry/18459 (accessed on 07 February 2026).
Yamasaki T. Subtle Daily Behavioral Changes and Mild Cognitive Impairment. Encyclopedia. Available at: https://encyclopedia.pub/entry/18459. Accessed February 07, 2026.
Yamasaki, Takao. "Subtle Daily Behavioral Changes and Mild Cognitive Impairment" Encyclopedia, https://encyclopedia.pub/entry/18459 (accessed February 07, 2026).
Yamasaki, T. (2022, January 19). Subtle Daily Behavioral Changes and Mild Cognitive Impairment. In Encyclopedia. https://encyclopedia.pub/entry/18459
Yamasaki, Takao. "Subtle Daily Behavioral Changes and Mild Cognitive Impairment." Encyclopedia. Web. 19 January, 2022.
Copy Citation
Short-term memory impairment, disorientation, and visuospatial deficits are the main symptoms in patients with Mild Cognitive Impairment (MCI) and very mild Alzheimer’s disease (AD). Interestingly, patients with MCI and very mild AD have subtle changes in their daily behavioral patterns alongside these main symptoms. Thus, subtle changes in daily behavioral patterns may be an indicator of early MCI and AD detection.
mild cognitive impairment
Alzheimer’s disease
nonwearable sensor-based in-home assessment
daily behavior
digital technologies
1. Changes in Daily Bahavioral Patterns Observed in MCI and AD Based on Performance- and Questionnarie-Based Assessments
Many reports evaluating daily behavioral changes in individuals with MCI and AD using performance-based assessments or informant-based or self-assessment questionnaires have been noted [1]. Regarding the performance-based instruments, the Direct Assessment of Functional Status was the best measure for detecting differences in global instrumental ADL functioning between MCI and healthy controls [1]. This measure is a standardized observation-based checklist designed to assess the functional capabilities of adults with AD, dementia, and schizophrenia. The examiner needs the evaluation form, pen or pencil, and ADL materials for testing. Simulated daily tasks are observed in the seven following areas: time orientation, communication, transportation, finance, shopping, grooming, and eating [2]. Using the Direct Assessment of Functional Status, Pereira et al. [3] found that patients with MCI performed significantly worse than healthy controls and better than patients with AD. Financial and shopping skills were the items that differentiated patients with MCI from healthy controls.
For the informant-based questionnaire, the Alzheimer’s Disease Cooperative Study scale for ADL in MCI seems to be a useful tool for global instrumental ADL assessment [1]. This questionnaire assesses the competence of patients with MCI in basic and instrumental ADL (covering 18 areas). It can be completed by a caregiver in a questionnaire format or administered by a clinician/researcher as a structured interview with caregivers [4]. Moreover, Perneczky et al. [5] used this questionnaire for measuring instrumental ADL in MCI. The overall score of this scale was significantly lower in the MCI group where the impaired ADL (14 out of 18 activities) were found. Activities involving memory or complex reasoning were particularly impaired, whereas more basic activities were unimpaired. In another paper, Perneczky et al. [6] examined whether this scale could be a significant predictor of the MCI diagnosis. They demonstrated that this scale discriminated well between patients and healthy controls with a sensitivity and specificity of 0.89 and 0.97, respectively, using receiver operator curve analysis.
The Seoul-Instrumental ADL and Lawton and Brody’s Instrumental ADL were used for the self-assessment questionnaires [7][8]. For example, Pérès et al. [8] assessed for instrumental ADL (telephone, transport, medication, and finances) in patients with MCI and dementia using Lawton and Brody’s Instrumental ADL. Patients with MCI were more frequently instrumental ADL-restricted (34.3%) than healthy controls (5.4%) but less than those with dementia (91.1%). Interestingly, the instrumental ADL-restricted subjects with MCI were more likely to develop dementia in >2 years (30.7%) than those with non-instrumental ADL-restricted MCI (7.8%) [8]. In addition, the odds ratios for dementia were 7.4 and 2.8 in instrumental ADL-restricted and non-instrumental ADL-restricted MCI, respectively, compared with healthy controls [8].
The instrumental ADL deficits were also analyzed between MCI subtypes. Moreover, MCI can be classified according to the presence/absence of episodic memory impairments (amnestic or non-amnestic) and the number of affected cognitive domains (single or multiple domains) [9]. A systematic review exhibited that the instrumental ADL deficits tended to be more pronounced in amnestic MCI than in non-amnestic MCI. The instrumental ADL deficits were more pronounced in the multiple-domain MCI than in the single-domain MCI [1].
Overall, changes in daily behavior are likely to be consistently present even in individuals with MCI in both the performance- and questionnaire-based methods. Furthermore, patients with MCI with instrumental ADL deficits seem to have a higher risk of converting to dementia than patients without ADL deficits. Thus, assessment of daily behavior (in particular, the instrumental ADL) is useful for early MCI detection and prognosis prediction.
Concerning the nature of changes in daily behavioral patterns (instrumental ADL deficits), Bruderer-Hofstetter et al. [10] recently developed a comprehensive model of ADL functioning that depicts the relevant influencing factors. In their studies, various factors are thought to be involved in these functional changes in patients with MCI. The relevant influencing factors include five cognitive factors (i.e., memory, attention, executive function, and two executive function subdomains (problem solving/reasoning and organization/planning)), five physical factors (i.e., seeing functions, hearing functions, balance, gait/mobility functions, and functional mobility functions), two environmental factors (i.e., social network/environment and support of social network/environment), and one personal factor (i.e., education) [10].
2. Changes in Daily Behavioral Patterns in MCI and AD Based on Nonwearable Sensor-Based In-Home Assessment
2.1. Digital Technologies for Monitoring of Daily Behavioral Patterns
In general, two main categories of sensors (i.e., wearable and nonwearable sensors) are used to monitor human behavior [11][12][13]. The two kinds of sensors have been used extensively in various systems. Wearable sensors are usually attached to a person directly (e.g., bracelet or cardio sensors) or to their clothes (e.g., an accelerometer or a step counter) to measure location, pulse rate, body temperature, blood pressure, and other important metrics as well as motion characteristics [11]. Conversely, nonwearable sensors are usually deployed in stationary locations of a house or a room and can detect a person and his movements and activities. Nonwearable sensors can specify the operational status of objects, measure water flow, room temperature, or door/cupboard opening/closings [11]. The types and characteristics of nonwearable sensors are summarized in Table 1.
Table 1. Types and characteristics of nonwearable sensors.
| Sensor | Measurement Type | Characteristics |
|---|---|---|
| Infrared sensors | Motion | - Most frequently used nonwearable sensors - Discover human presence in a room - Detect motion in a specific area - Locate a human within a house |
| Ultrasonic sensors | Motion | - Person detection and localization by measuring distances to objects |
| Photoelectric sensors | Motion | - Detect a light source and output a signal |
| Vibration sensors | Vibration | - Detect a person falling, interaction with various objects, flushing toilets, and water flows |
| Pressure sensors | Pressure on object | - Detect the presence of a person, steps, and fall events - Deploy in the form of floor mats and smart tiles |
| Magnetic switches | Opening or closing | - Detect opening and closing of doors or cupboards - Provide information on users accessing particular rooms and opening dressers, refrigerators, or trash cans |
| Audio sensors | Activity-related sound | - Detect sounds in a house - Discriminate between different types of sounds |
| Wattmeter and other sensors | Consumption information | - Measure electricity consumption of domestic appliances and light |
Wearable sensors have the advantage of higher localization accuracy and tracking. Additionally, wearable sensors are much more available and provide a timely and economical fashion for detecting MCI and AD compared with nonwearable sensors [11]. However, wearable sensor-based monitoring is more intrusive and demands that older adults with various degrees of cognitive levels to remember wear the devices as well as the need for regular charging of the devices [14]. In contrast, nonwearable sensors are less intrusive and can monitor activities in a real life and naturalistic environment without causing any interference to an individual’s daily routines [14]. From these characteristics, it seems that nonwearable sensors are more suitable for the monitoring and detection of patients with MCI and AD. Thus, we focused on research using nonwearable sensor-based in-home assessment.
2.2. Changes in Daily Bahavioral Patterns Observed in MCI and AD Based on Nonwearable Sensor-Based In-Home Assessment
Several studies have investigated one of the daily behaviors (e.g., walking, sleeping, and going out) in patients with MCI and AD (Table 2). For example, Hayes et al. [15] investigated the walking speed and daily activity of patients with MCI and healthy elderly people living independently and alone in the community using unobtrusive sensors (passive infrared motion sensors for each room and magnetic contact door sensors for each door) at home for at least 6 months. Walking speed was more variable in the MCI group than in the healthy group. In addition, the day-to-day activity pattern was more variable in patients with MCI. Dodge et al. [16] also examined in-home walking speeds using passive infrared sensors fixed in a series on the ceiling of homes of patients with amnestic MCI and non-amnestic MCI and healthy controls for >3 years. Patients with non-amnestic MCI were characterized by a reduced walking speed. Furthermore, two distinct trajectories (the highest and lowest variability) were predominantly associated with non-amnestic MCI. These studies suggest the alteration of walking behavior in patients with MCI. Moreover, Hayes et al. [17] explored the relationship between sleep disturbances and patients with MCI in community-dwelling seniors using wireless passive infrared motion sensors in each room of the home (bedroom, bathroom, kitchen, living room, and hallway-entry areas) and magnetic contact door sensors for each door over 6 months. Consequently, patients with amnestic MCI and non-amnestic MCI, and cognitively intact volunteers showed different patterns of sleep disturbance-. In particular, patients with amnestic MCI had less disturbed sleep than both those with non-amnestic MCI and healthy subjects. These differences in sleep disruption between amnestic MCI and non-amnestic MCI may be related to differences in the pathology underlying these MCI subtypes. Petersen et al. [18] investigated the relationship between time out-of-home and cognitive status, physical ability, and emotional state in patients with MCI and healthy elderly using pyroelectric infrared motion sensors in each room and contact sensors on the refrigerator and doors of the home for up to 1 year. They found that cognitive status was significantly associated with time out-of-home. Furthermore, patients with MCI spent an average of 1.67 h more inside the home than healthy elderly.
Recent studies focused on changes in daily behavioral patterns in general, rather than just one daily activity (Table 2). Urwyler et al. [19] investigated differences in daily behavioral pattern performance between patients with dementia and healthy controls using unobtrusive sensors for 20 consecutive days. An unobtrusive sensor network comprising 10 wireless sensor boxes was installed in the home (Figure 1). Each sensor box consisted of five sensors (temperature, humidity, luminescence, presence (passive infrared radiation), and acceleration). Consequently, a significant difference in daily behavioral patterns was observed between patients with dementia and healthy controls. Specifically, patients with dementia revealed unorganized behavior patterns (Figure 2). Rawtaer et al. [20] examined changes in behaviors in patients with MCI using the multiple sensor system (passive infrared motion sensors, proximity beacon tags, a sensor-equipped medication box, a bed sensor, and a wearable sensor) for >2 months. Patients with MCI were less active than healthy subjects and had more sleep interruptions per night. In addition, patients with MCI had forgotten their medications more times per month than healthy subjects. Overall, changes in various kinds of daily behavioral functions were observed even in patients with MCI.
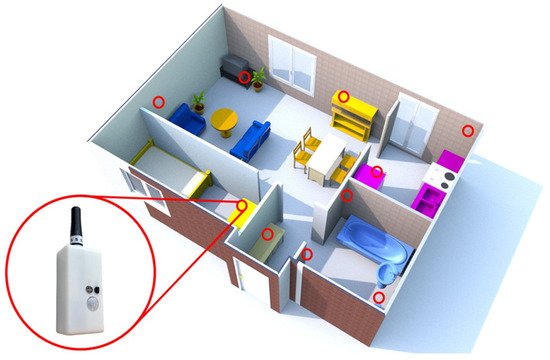
Figure 1. Floor plan of an apartment showing the placement of sensor boxes (red circles). The figure is adapted from Urwyler et al. [19] (CC BY 4.0).
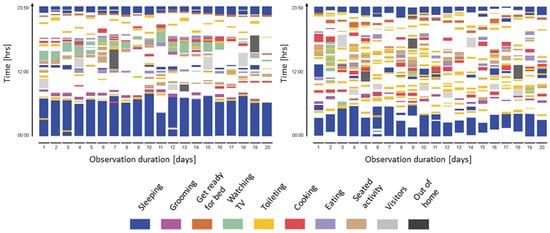
Figure 2. Activity maps of a healthy control (left) and a patient with dementia (right) visualized from data continuously measured for 20 consecutive days. Activity maps of patients with dementia reveal unorganized behavior patterns, and heterogeneity differed significantly between the healthy control and the patient. The figure is adapted from Urwyler et al. [19] (CC BY 4.0).
| References | Participants and Study Protocol (1. Study Design; 2. Participants; 3. Sensor Type; 4. Duration; 5. Machine Learning Technique) |
Main Findings |
|---|---|---|
| Hayes et al. [26] |
|
- Walking speed was more variable in patients with MCI. - Day-to-day pattern of activities was more variable in patients with MCI. |
| Dodge et al. [27] |
|
- Daily walking speeds and their variability were associated with non-amnestic MCI. |
| Hayes et al. [28] |
|
- Patients with amnestic MCI showed less sleep disturbance than both those with non-amnestic MCI and healthy elderly. |
| Petersen et al. [29] |
|
- Patients with MCI spent an average 1.67 h more inside the home than healthy elderly. |
| Urwyler et al. [30] |
|
- Patients with dementia showed unorganized behavior patterns. |
| Rawtaer et al. [31] |
|
- Patients with MCI were less active than healthy subjects and had more sleep interruptions per night. - Patients with MCI had forgotten their medications more times per month than healthy subjects. |
| Akl et al. [34] |
|
- Variabilities in weekly walking speed, morning and evening walking speeds, and subjects’ age and gender were the most important for the process of detecting MCI. - This study autonomously detected MCI with receiver operating characteristic curve (0.97) and precision–recall curve (0.93) using a time windows of 24 weeks. |
| Akl et al. [35] |
|
- This study automatically detected MCI (F0.5 score, 0.856) and non-amnestic MCI (F0.5 score, 0.958). |
| Alberdi et al. [36] |
|
- Sleep and overnight patterns along with daily routine features contributed to the prediction of several health assessments. - All algorithms could build statistically significant prediction models. |
| Nakaoku et al. [37] |
|
- Three independent power monitoring parameters (air conditioner, microwave oven, and induction heater) representing activity behavior were associated with cognitive impairment. - The prediction model with power monitoring data had better predictive ability (accuracy, 0.82; sensitivity, 0.48; and specificity, 0.96). |
ADL activities of daily living, MCI mild cognitive impairment, AD Alzheimer’s disease.
2.3. Machine Learning–Based Prediction Model for Detecting Individuals with MCI
Machine learning is a subdiscipline of artificial intelligence and has been extensively used in recent studies to predict behavioral/cognitive abnormalities utilizing sensor-based activity data [21][22]. Several studies have been noted to apply these methods to MCI differentiation [23][24][25][26] . Moreover, support vector machine and random forest were the most commonly used techniques, although a wide variety of machine learning techniques were employed. Several metrics are used to evaluate prediction models (e.g., area under the receiver operator characteristic and precision–recall curves) and the F-score [21][22][23][24][25][26].
ADL activities of daily living, MCI mild cognitive impairment, AD Alzheimer’s disease.
Akl et al. [23] explored the ability of signal processing along with machine learning algorithms to autonomously detect MCI using home-based unobtrusive sensing technologies (passive infrared motion sensors in rooms, wireless contact switches on doors, and motion sensors on the ceiling). They found that variabilities (i.e., weekly walking speed, morning and evening walking speeds, and subjects’ age and gender) were the most important factors in the process of detecting MCI. The authors autonomously detected MCI with receiver operator characteristic and precision–recall curves of 0.97 and 0.93, respectively, using a time window of only 24 weeks [23]. A clustering-based method to automatically detect MCI using estimated generalized linear models of their home activity was proposed by Akl et al. in another study [24]. Continuous monitoring was conducted via unobtrusive sensing technologies (passive infrared motion sensors in the room and wireless contact switches on the doors). The authors automatically detected MCI and non-amnestic MCI with F0.5 scores of 0.856 and 0.958, respectively [24]. Moreover, Alberdi et al. [25] assessed the possibility of detecting changes in psychological, cognitive, and behavioral symptoms of MCI by making use of unobtrusively collected smart home behavior data and machine learning techniques. They found that sensor-based activity observations (e.g., sleep and overnight patterns) and daily routine features contributed significantly to the prediction of several health assessments. All algorithms could build statistically significant prediction models [25]. In addition, Nakaoku et al. [26] investigated whether unobtrusive in-house power monitoring technologies could be used to predict cognitive impairment. Daily activity data were collected using a well-established unobtrusive in-house power monitoring system installed in the participants’ homes. Several electric appliances (air conditioner, microwave oven, washing machine, rice cooker, television, and induction heater) were monitored. Three independent power monitoring parameters (air conditioner, microwave oven, and induction heater) representing activity behavior were associated with cognitive impairment. Regarding the prediction models for cognitive impairment, the model with power monitoring data had a better predictive ability (accuracy, 0.82; sensitivity, 0.48; and specificity, 0.96) than the model without power monitoring data (accuracy, 0.76; sensitivity, 0.30; and specificity, 0.95) [26]. From the findings of these studies, combining data collection by sensors and machine learning are useful to detect patients with MCI.
References
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.J.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Grassel, E.; et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Res. Ther. 2015, 7, 17.
- Gary, K.W. Direct Assessment of Functional Status. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011.
- Pereira, F.S.; Yassuda, M.S.; Oliveira, A.M.; Diniz, B.S.; Radanovic, M.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Profiles of functional deficits in mild cognitive impairment and dementia: Benefits from objective measurement. J. Int. Neuropsychol. Soc. 2010, 16, 297–305.
- Alzheimer’s Disease Co-operative Study ADL Scale for Mild Cognitive Impairment (ADCS-ADL-MCI). Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011.
- Perneczky, R.; Pohl, C.; Sorg, C.; Hartmann, J.; Tosic, N.; Grimmer, T.; Heitele, S.; Kurz, A. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int. J. Geriatr. Psychiatry 2006, 21, 158–162.
- Perneczky, R.; Pohl, C.; Sorg, C.; Hartmann, J.; Komossa, K.; Alexopoulos, P.; Wagenpfeil, S.; Kurz, A. Complex activities of daily living in mild cognitive impairment: Conceptual and diagnostic issues. Age Ageing 2006, 35, 240–245.
- Kim, K.R.; Lee, K.S.; Cheong, H.K.; Eom, J.S.; Oh, B.H.; Hong, C.H. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 27, 278–285.
- Pérès, K.; Chrysostome, V.; Fabrigoule, C.; Orgogozo, J.M.; Dartigues, J.F.; Barberger-Gateau, P. Restriction in complex activities of daily living in MCI: Impact on outcome. Neurology 2006, 67, 461–466.
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992.
- Bruderer-Hofstetter, M.; Sikkes, S.A.M.; Münzer, T.; Niedermann, K. Development of a model on factors affecting instrumental activities of daily living in people with mild cognitive impairment—A Delphi study. BMC Neurol. 2020, 20, 264.
- Debes, C.; Merentitis, A.; Sukhanov, S.; Niessen, M.; Frangiadakis, N.; Bauer, A. Monitoring activities of daily living in smart homes: Understanding human behavior. IEEE Signal. Process. Mag. 2016, 33, 81–94.
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/wearable devices opportunity. Npj Digit. Med. 2019, 2, 9.
- Eisa, S.; Moreira, A. A behaviour monitoring system (BMS) for ambient assisted living. Sensors 2017, 17, 1946.
- Narasimhan, R.; Muthukumaran, G.; McGlade, C. Current state of non-wearable sensor technologies for monitoring activity patterns to detect symptoms of mild cognitive impairment to Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2021, 2021, 2679398.
- Hayes, T.L.; Abendroth, F.; Adami, A.; Pavel, M.; Zitzelberger, T.A.; Kaye, J.A. Unobtrusive assessment of activity patterns associated with mild cognitive impairment. Alzheimer’s Dement. 2008, 4, 395–405.
- Dodge, H.H.; Mattek, N.C.; Austin, D.; Hayes, T.L.; Kaye, J.A. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology 2012, 78, 1946–1952.
- Hayes, T.L.; Riley, T.; Mattek, N.; Pavel, M.; Kaye, J.A. Sleep habits in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2014, 28, 145–150.
- Petersen, J.; Austin, D.; Mattek, N.; Kaye, J. Time out-of-home and cognitive, physical, and emotional wellbeing of older adults: A longitudinal mixed effects model. PLoS ONE 2015, 10, e0139643.
- Urwyler, P.; Stucki, R.; Rampa, L.; Müri, R.; Mosimann, U.P.; Nef, T. Cognitive impairment categorized in community-dwelling older adults with and without dementia using in-home sensors that recognise activities of daily living. Sci. Rep. 2017, 7, 42084.
- Rawtaer, I.; Mahendran, R.; Kua, E.H.; Tan, H.P.; Tan, H.X.; Lee, T.S.; Ng, T.P. Early detection of mild cognitive impairment with in-home sensors to monitor behavior patterns in community-dwelling senior citizens in Singapore: Cross-sectional feasibility study. J. Med. Internet Res. 2020, 22, e16854.
- Sajda, P. Machine learning for detection and diagnosis of disease. Annu. Rev. Biomed. Eng. 2006, 8, 537–565.
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281.
- Akl, A.; Taati, B.; Mihailidis, A. Autonomous unobtrusive detection of mild cognitive impairment in older adults. IEEE Trans. Biomed. Eng. 2015, 62, 1383–1394.
- Akl, A.; Chikhaoui, B.; Mattek, N.; Kaye, J.; Austin, D.; Mihailidis, A. Clustering home activity distributions for automatic detection of mild cognitive impairment in older adults. J. Ambient. Intell. Smart Environ. 2016, 8, 437–451.
- Alberdi, A.; Weakley, A.; Schmitter-Edgecombe, M.; Cook, D.J.; Aztiria, A.; Basarab, A.; Barrenechea, M. Smart home-based prediction of multi-domain symptoms related to Alzheimer’s disease. IEEE J. Biomed. Health Inform. 2018, 22, 1720–1731.
- Nakaoku, Y.; Ogata, S.; Murata, S.; Nishimori, M.; Ihara, M.; Iihara, K.; Takegami, M.; Nishimura, K. AI-assisted in-house power monitoring for the detection of cognitive impairment in older adults. Sensors 2021, 21, 6249.
More
Information
Subjects:
Geriatrics & Gerontology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
928
Revisions:
3 times
(View History)
Update Date:
21 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




