The aim of this entry was to assess the effect of elevated temperature on the growth, morphology and spatial orientation of lupine roots at the initial stages of development and on the formation of lupine root architecture at later stages. Two lupine species were studied—the invasive Lupinus polyphyllus Lindl. and the non-invasive L. luteus L. The plants were grown in climate chambers under 25 °C and simulated warming at 30 °C conditions. The angle of root curvature towards the vector of gravity was measured at the 48th hour of growth, and during a 4-h period after 90° reorientation. Root biometrical, histological measurements were carried out on 7-day-old and 30-day-old plants. The elevation of 5 °C affected the root formation of the two lupine species differently. The initial roots of L. polyphyllus were characterized by worse spatial orientation, reduced growth and reduced mitotic index of root apical meristem at 30 °C compared with 25 °C. The length of primary roots of 30-day-old lupines and the number of lateral roots decreased by 14% and 16%, respectively.
1. Introduction
The world is experiencing ongoing global climate change, which can have serious consequences on plants, including changes in the availability of certain nutrients. For understanding the effects of climate warming on plant root systems, particularly their spatial distribution, it is essential to predict plant performance and community recovery in a warming climate. Compared with shoots, much less is known about how roots, especially root system architecture (RSA), may respond to elevated temperature. In addition, limited information is available on the specificities of the effects of elevated temperatures on the development of the root system in invasive plants. How does an increase in temperature change the intensity and the direction of root formation? To answer this question, researchers have compared the responses of plants with different RSAs in their studies
[1][2]. The ability of a plant to take up nutrients is closely associated with the size and morphology of its root system
[1][3]. Any changes in the growth or morphological modifications of root systems may provoke undesirable consequences in nutrient uptake
[4]. It is recognized that many aspects of plant metabolism are accelerated by elevated temperatures
[5][6]. Other environmental factors such as water, nutrients and temperature also have a strong influence on root structure
[7]. Roots need an optimal temperature range to have a proper growth rate and function. In general, the optimal root temperature tends to be lower than the optimal shoot temperature
[8][9]. It is evident that the optimum root temperature of plants varies depending on the species. Within this range, higher temperatures are generally associated with modified root-to-shoot ratios, while further increases in temperature would reduce root development and cause a change in RSA, thus reducing the root-to-shoot ratio
[10]. For instance, some plants tend to produce more extensive root systems in elevated temperatures. An increase in temperature slows down lateral root growth in adult maize plants and promotes the development of long axial roots to reach deeper soil layers for water
[11][12]. However, in potatoes, the initiation and elongation of adventitious and lateral roots were inhibited by increasing temperature. Another effect of warmer soil on potatoes is the swelling of the root cap meristem and the bending of the root tip. The alteration of root growth in these plants appeared due to a reduced rate of cell division
[13][14]. Similarly, in sorghum, the high root zone temperature reduced the rate of root elongation and cell production rate
[15]. The response of RSA to elevated temperature can be species-specific, as different species often have different optimum temperatures for root growth
[16][17]. Literature data show that the effect of increasing temperature on root growth of plant seedlings can be promotive, inhibitive or first promotive then inhibitive after an optimum temperature is reached
[18][19]. Even for species sharing the same habitat, their RSA can have species-specific responses to increased temperature
[20]. Differences in the RSA of plant species may determine the intensity and direction of root formation in response to elevated temperatures. At high temperatures, the negative root response may have been intensified, with a competitive advantage going to species with larger and more rapidly forming roots.
Literature data indicate that greater root resilience plays a key role in plants adapting to high temperatures
[21][22][23] in all stages of root development, including tropisms and the formation of new organs
[24][25]. Furthermore, the oriented plant growth, which is collectively referred to as tropism
[26][27], is influenced by various environmental factors, such as light, temperature, water and gravity. Gravitropism is an important tropic response that triggers asymmetric cell elongation in plant organs in response to gravity. It proceeds through three sequential steps: gravity perception, signal transduction and asymmetric cell elongation in the responding plant organs
[28][29]. The roots grow downward, and the shoots grow upward, showing positive and negative gravitropic responses, respectively
[30][31]. The well-known Cholodny-Went hypothesis illustrates that gravitropic stimuli result in differential cell elongation in the responding organs
[32][33][34][35]. It has been shown that gravitropic perception occurs in the columella cells in the roots upon gravity stimulation
[36][37]. The gravitropic response of plant organs is influenced by a variety of environmental signals. The best understood are the effects of light and temperature. Many scientists agree that climate change will alter habitat biodiversity and increase vulnerability to invasion. However, there is little information on the impact of potentially increasing global temperatures on the growth and development of alien plant species at the early stages of development. Moreover, one of the selected lupine species is invasive in Lithuania,
L. polyphyllus, and there is very limited information on the specificities of the effect of elevated temperatures on the root system development of invasive plants. Therefore, in this research, RSA traits of seedlings of two lupine species (
L. polyphyllus and
L. luteus) with different spreading performances for understanding their responses to temperature change were studied. We hypothesized that increased temperature may differentially affect root growth, spatial orientation and root architecture of non-native plant species, thereby influencing them to become invasive. Studies on plant root system adaptive responses to altered temperature can provide the knowledge needed for the efficient management of invasive species. Thus, the goal of the current study was to investigate root growth, morphology and spatial orientation of two alien lupine species during the early growth stage at the elevated temperature.
2. The Initial Root Growth at 25 °C and 30 °C
2.1. Angle of Curvature of Initial Roots at 25 °C and 30 °C
After 48 h of seedling growth, the spatial orientation and growth direction of the roots of both lupines depended on the temperature: the angle of curvature of the primary roots of the invasive L. polyphyllus with respect to the gravitational vector was 6.2° at 25 °C, and 20.8° at 30 °C. The initial roots of the non-invasive L. luteus showed a better orientation towards the gravity vector at 30 °C (Table 1, Figure 1).
Figure 1. Spatial orientation of the initial roots of L. polyphyllus and L. luteus seedlings at 25 °C and 30 °C after 48 h. Scale bar, 10 mm.
Table 1. Influence of 25 °C and 30 °C temperatures on the angle of curvature of the initial roots of L. polyphyllus and L. luteus seedlings grown vertically for 48 h.
| Plant Species |
Lupinus polyphyllus |
Lupinus luteus |
| Temperature |
25 °C |
30 °C |
25 °C |
30 °C |
| Angle of curvature, degrees |
6.2 ± 0.53 a |
20.8 ± 0.95 b |
14.2 ± 1.21 c |
6.8 ± 0.43 a |
2.2. Gravitropic Response of Initial Roots to 90° Reorientation
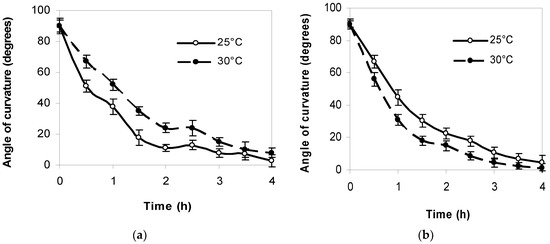
The strongest root response to gravitropic irritation in both lupine species was found to occur within the first hour. The gravitropic bending of L. polyphyllus roots after 1 h was 16° greater at 25 °C than at 30 °C. The gravitropic bending of L. luteus roots was more intensive at 30 °C. The gravitropic response of the roots of both lupine species to a 90° reorientation was closer to the direction of gravity after 4 h (Figure 2).
Figure 2. The dynamics of gravitropic response of L. polyphyllus (a) and L. luteus (b) roots to 90° reorientation at 25 °C and 30 °C.
2.3. Growth of Primary Roots of 7-Day-Old Seedlings at 25 °C and 30 °C
Morphometric studies showed that the length of roots of the invasive lupine grown at 30 °C for seven days was approximately 12% lower than that of the plants grown at 25 °C (Figure 3), while the roots of the non-invasive lupine grew up to 13% longer at 30 °C.
Figure 3. Effect of 25 °C and 30 °C temperature on root growth parameters of seven-day-old seedlings of two lupine species. Scale bar, 10 mm.
We found that the root-to-shoot ratio of both species decreased at 30 °C (Table 2). This index, in the case of L. polyphyllus, decreased crucially by 65% and in the case of L. luteus by 22%.
Table 2. Influence of 25 °C and 30 °C temperature on root-to-shoot ratio of the seven-day-old seedlings of L. polyphyllus and L. luteus.
| Plant Species |
Lupinus polyphyllus |
Lupinus luteus |
| Temperature |
25 °C |
30 °C |
25 °C |
30 °C |
| Root-to-shoot ratio |
0.182 ± 0.03 a |
0.063 ± 0.01 b |
0.217 ± 0.03 a |
0.169 ± 0.01 a |
2.4. Root Apex Development at 25 °C and 30 °C
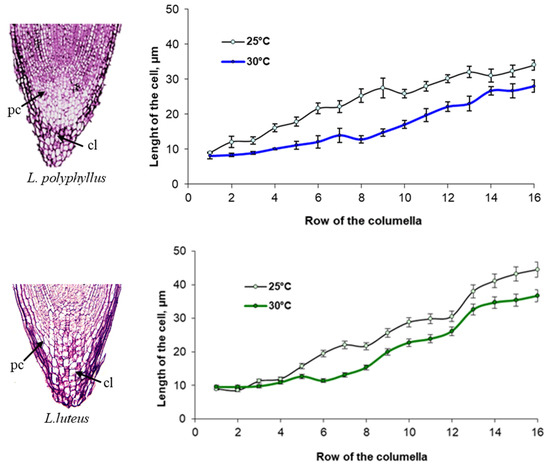
Cytomorphological analysis of the root cap columella of L. polyphyllus showed that the length of the cells in the individual rows of the columella varied with temperature (Figure 4). From the seventh row of the columella onwards, cell length increased more at 25 °C than at 30 °C. The changes in cell length in the L. luteus columella were substantially different from that of L. polyphyllus. The cell length of the columella at 30 °C was greater starting from the second row onwards. This trend was observed in all the following rows.
Figure 4. Impact of 25 °C and 30 °C temperature on the length of cells in the columella (cl) rows of the primary root cap (pc) (from the initial cells) of L. polyphyllus and L. luteus.
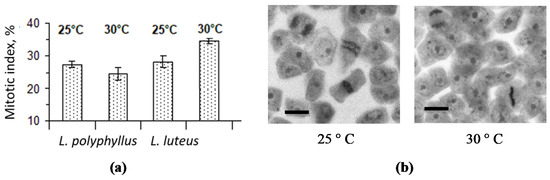
Determination of the cell division mitotic index (MI) in L. polyphyllus root apical meristem preparations showed that the cell MI value decreased by 12% in the test variant at 30 °C as compared with 25 °C (Figure 5a). Contrary, the calculation of MI in the non-invasive L. luteus root apical meristem indicated a significant increase at 30 °C. By observing the cross-sections of the invasive lupine root apex, we determined that meristem cells occurred in the prophase, metaphase and some even in the anaphase in the test variant at 25 °C, whereas most cells of plants grown at 30 °C were found in the prophase (Figure 5b).
Figure 5. Effect of 25 °C and 30 °C temperatures on L. polyphyllus and L. luteus root apex meristem cells mitotic activity (a), fragments of primary root apical meristem pressed preparations from L. polyphyllus seedlings (b). Scale bar, 20 μm.
3. The Development of 30-Day-Old Lupine Roots at 25 °C and 30 °C
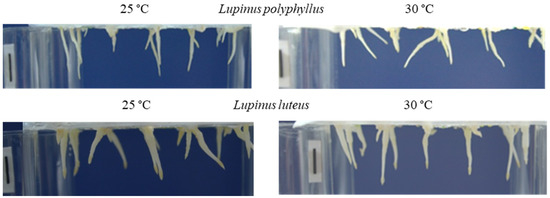
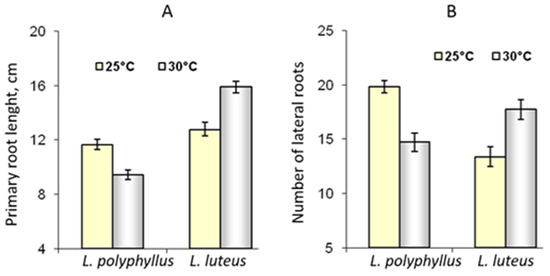
The data of the morphometric measurements showed that simulated 5 °C warming affected invasive Lupinus polyphyllus root formation:primary root length decreased by 14% and the number of lateral roots by 16%. The length of the primary root and the number of lateral roots of non-invasive L. luteus were higher at 30 °C (Figure 6 and Figure 7).
Figure 6. Effect of 25 °C and 30 °C temperature on the length of primary roots (A) and the number of lateral roots (B) of invasive L. polyphyllus and non-invasive L. luteus plants grown in soil for 30 days.
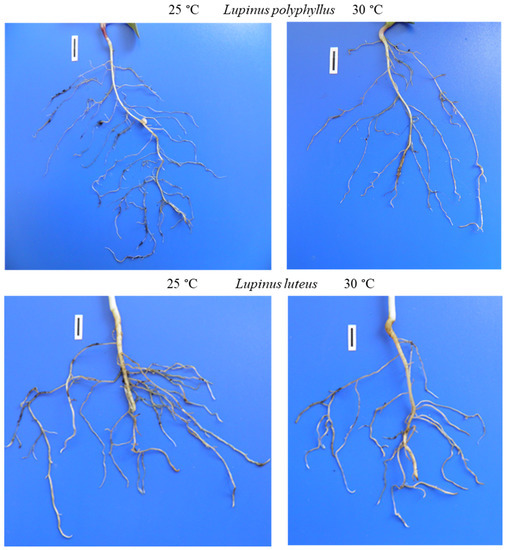
Figure 7. Roots of invasive L. polyphyllus and non-invasive L. luteus grown in the soil for 30 days. Scale bar, 10 mm.