
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abul Hossain | + 3996 word(s) | 3996 | 2022-01-13 03:14:48 | | | |
| 2 | Peter Tang | Meta information modification | 3996 | 2022-01-17 06:45:22 | | |
Video Upload Options
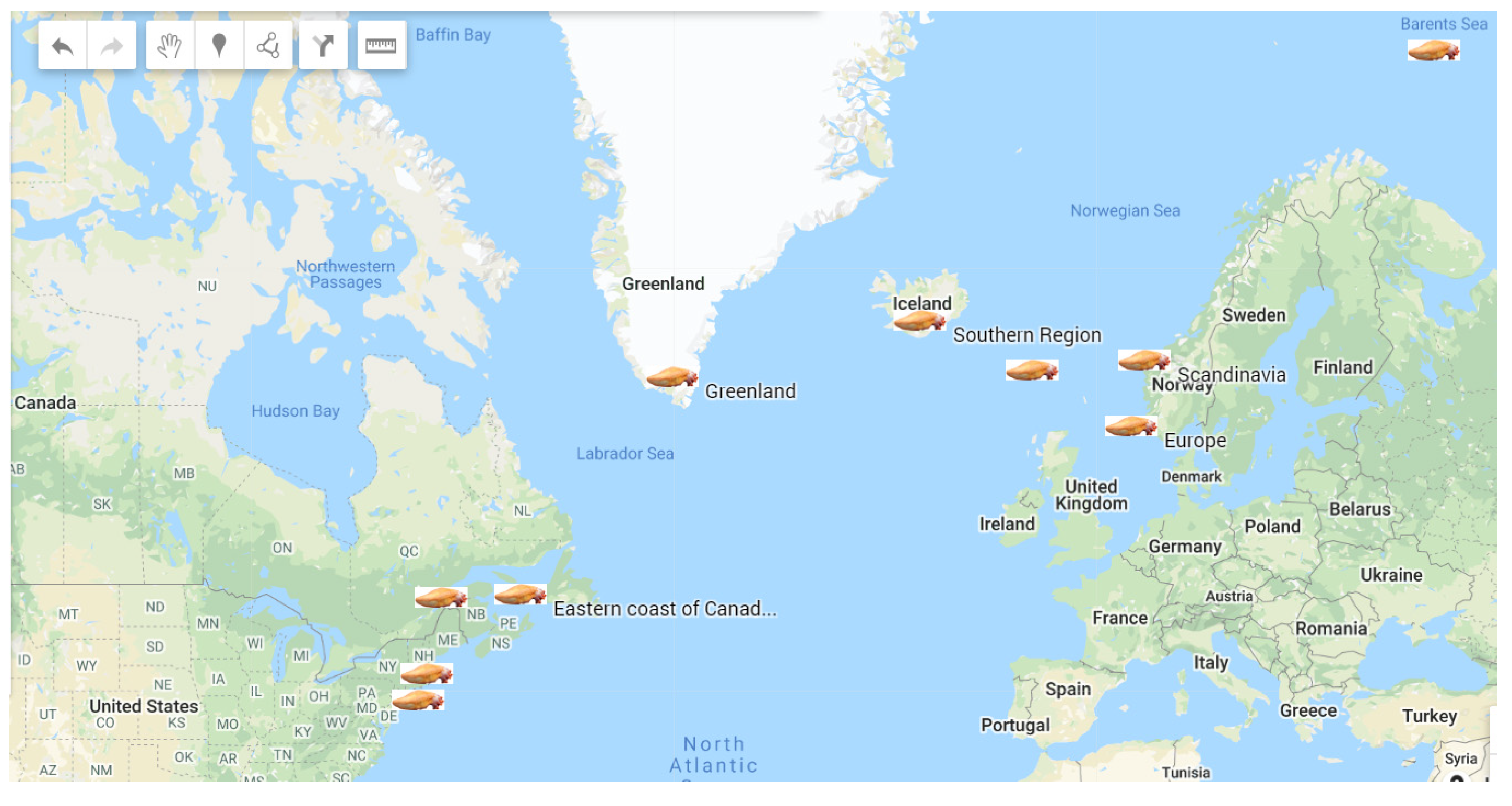
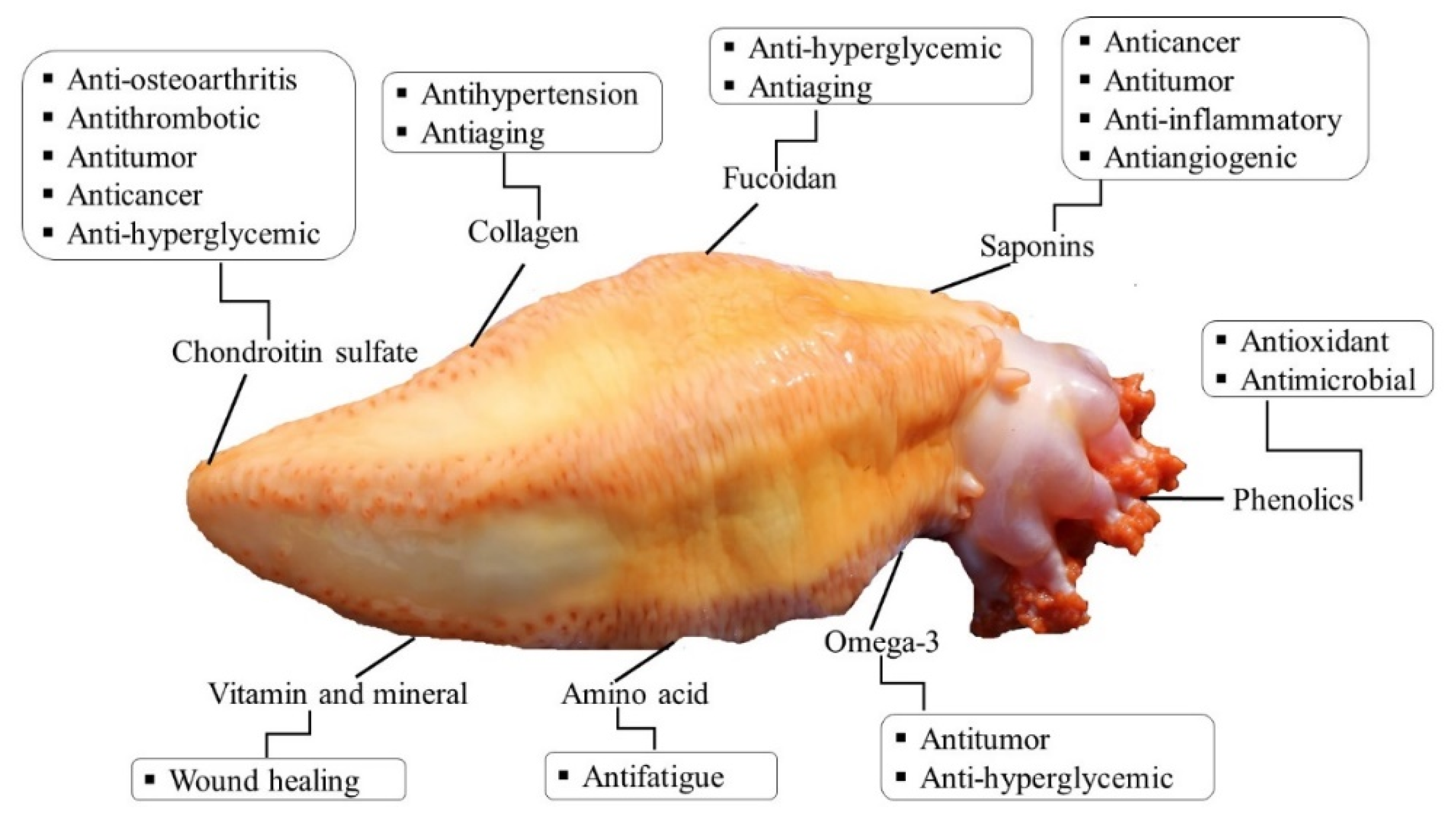
Sea cucumber (Cucumaria frondosa) is the most abundant and widely distributed species in the cold waters of North Atlantic Ocean. C. frondosa contains a wide range of bioactive compounds, mainly collagen, cerebrosides, glycosaminoglycan, chondroitin sulfate, saponins, phenols, and mucopolysaccharides, which demonstrate unique biological and pharmacological properties. In particular, the body wall of this marine invertebrate is the major edible part and contains most of the active constituents, mainly polysaccharides and collagen, which exhibit numerous biological activities, including anticancer, anti-hypertensive, anti-angiogenic, anti-inflammatory, antidiabetic, anti-coagulation, antimicrobial, antioxidation, and anti- osteoclastogenic properties.
1. Introduction
2. Description, Growth, and Distribution
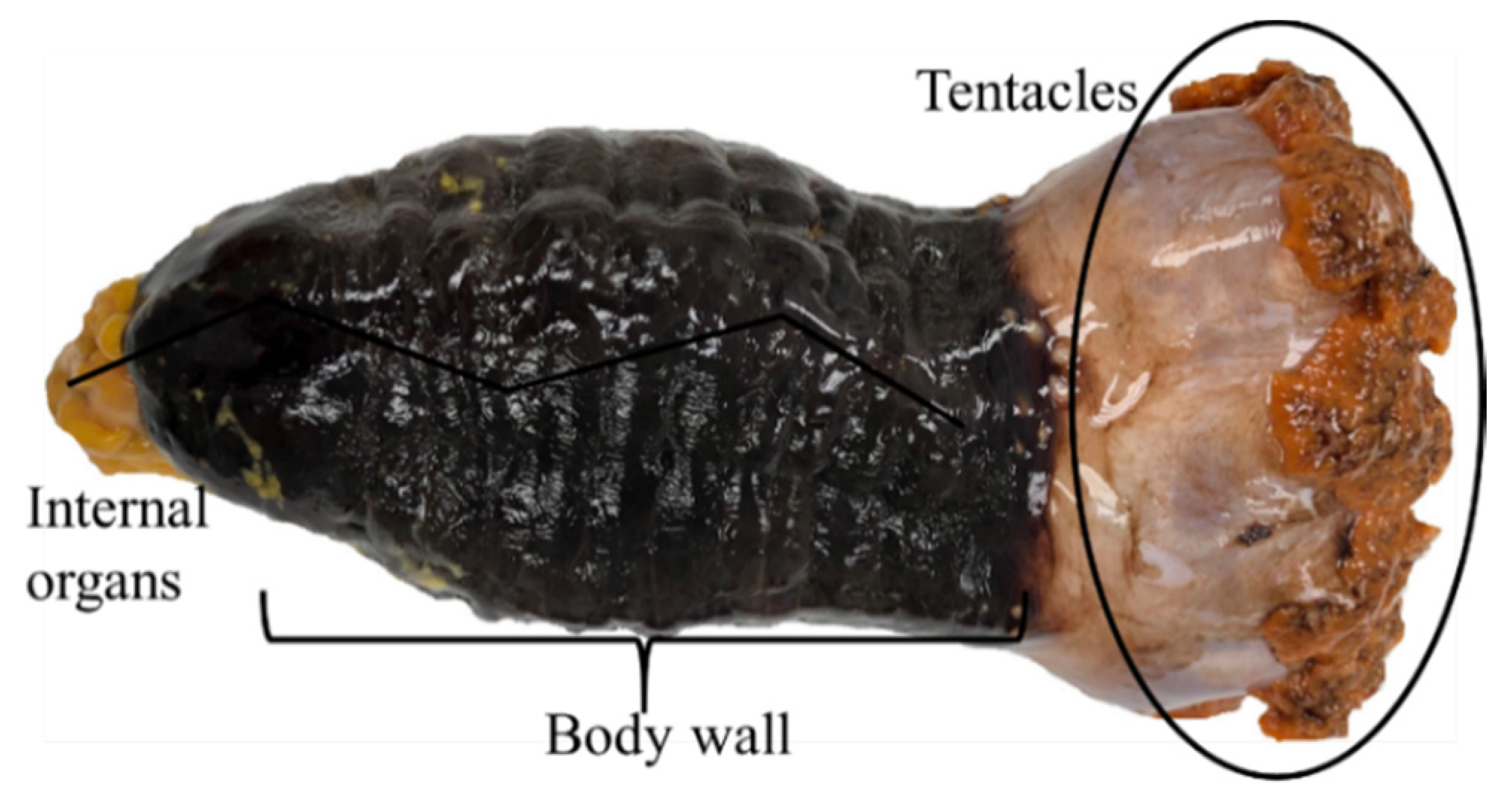
3. Proximate Composition
|
Amino acids |
Sea cucumber with viscera (mg/g) a |
Body wall (mg/g) a |
Viscera (%) b |
Fatty Acids |
Sea cucumber with viscera (%) a |
Body wall (%) a |
Viscera (%) b |
|---|---|---|---|---|---|---|---|
|
Valine |
16.8 |
19.7 |
5.4 |
14:00 |
1.8 |
1.88 |
10.1 |
|
Methionine |
9.4 |
10.3 |
2.3 |
15:00 |
4.03 |
2.18 |
0.3 |
|
Isoleucine |
12.6 |
13.9 |
4.7 |
16:00 |
2.83 |
2.33 |
13.3 |
|
Leucine |
28.3 |
30.8 |
7.2 |
16:1 n-7 |
7.36 |
5.75 |
6 |
|
Phenylalanine |
13.1 |
17.8 |
3.5 |
17:1 n-7 |
2.44 |
3.87 |
N/A |
|
Histidine |
3.3 |
2.8 |
2.3 |
18:00 |
4.2 |
5.41 |
2.1 |
|
Threonine |
10.9 |
12.9 |
5 |
18:1 n-9 |
3.72 |
2.43 |
4.9 |
|
Lysine |
30.6 |
29.1 |
6.6 |
18:1 n-7 |
3.37 |
3.52 |
N/A |
|
Aspartic acid |
27.8 |
39.1 |
10 |
20:1 n-9 |
1.66 |
4 |
N/A |
|
Glutamic acid |
57.5 |
66.4 |
14.3 |
20:3 n-3 |
2.54 |
5 |
2.5 |
|
Serine |
15.5 |
19.3 |
4.3 |
20:5 n-3 |
43.2 |
46.1 |
17.1 |
|
Glycine |
29.8 |
56.1 |
8 |
22:00 |
2.09 |
1.95 |
0 |
|
Alanine |
23.2 |
32 |
6.6 |
22:1 n-9 |
3.34 |
2.25 |
2.5 |
|
Arginine |
24.7 |
30.1 |
9.1 |
22:6 n-3 |
5.81 |
4.96 |
0.3 |
|
Proline |
17.3 |
24 |
4 |
20:2 n-6 |
N/A |
N/A |
2.2 |
|
Tyrosine |
13.4 |
15.6 |
5 |
20:4 n-6 |
N/A |
N/A |
10.4 |
a Zhong, Khan, and Shahidi [51]; b Mamelona, Saint-Louis, and Pelletier [52].
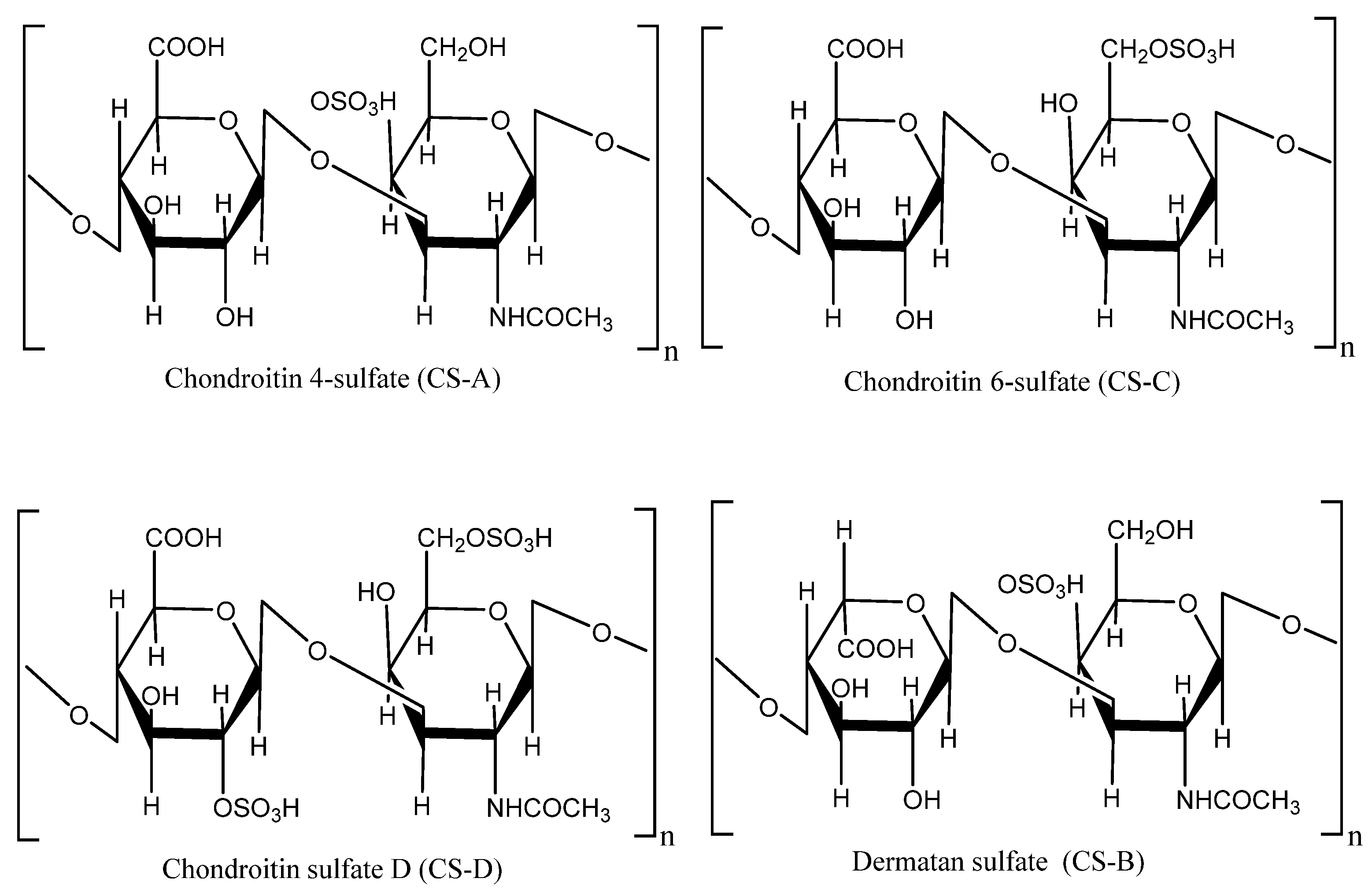
4. Bioactive Compounds and Methods of their Extraction and Isolation
4.1. Polysaccharides
4.2. Fucoidan
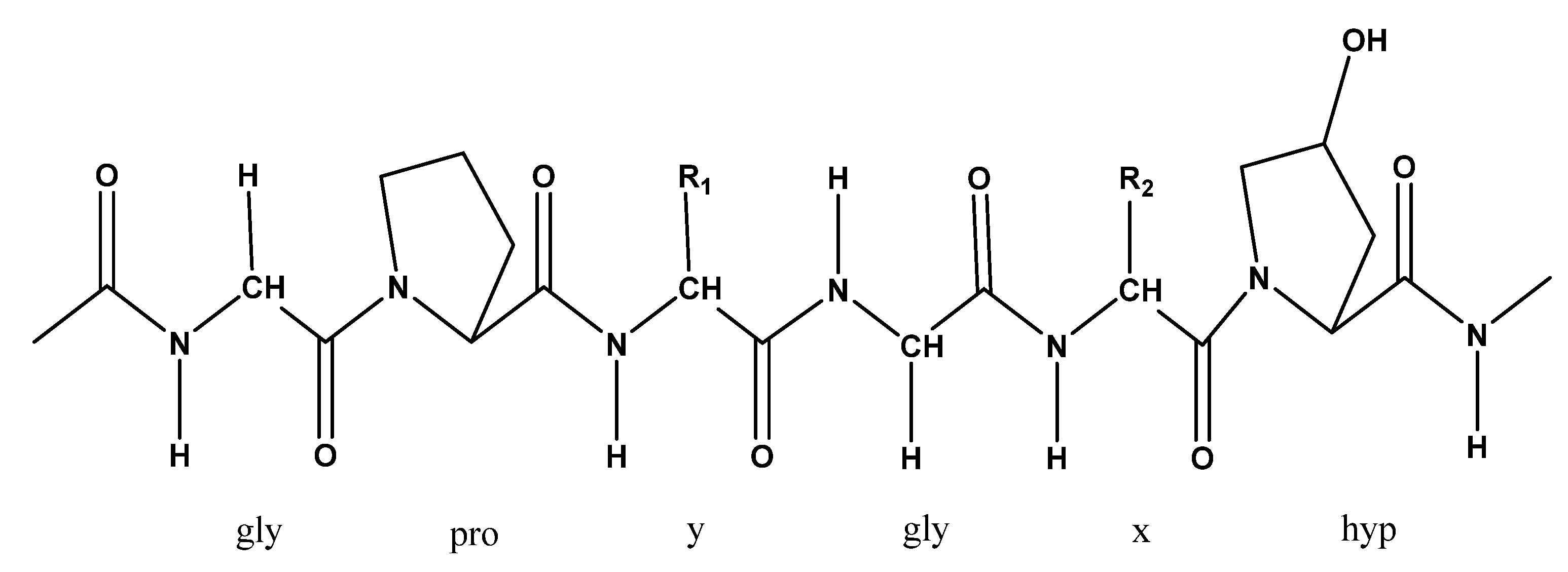
4.3. Collagen
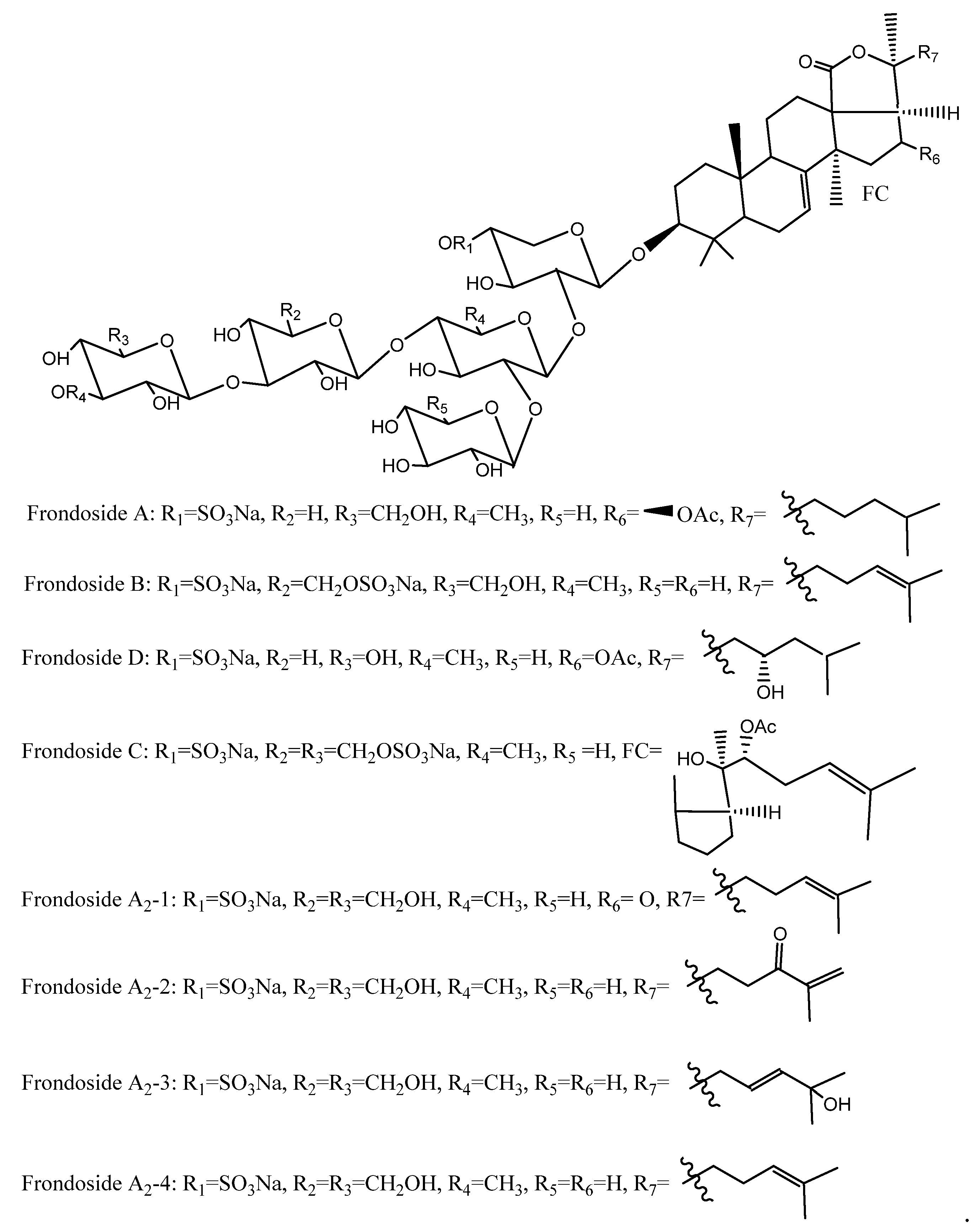
4.4. Saponins
4.5. Phenolic Compounds
5. Potential Biological Activities and Medicinal Effects
|
Bioactives |
Body Parts |
Biological and Medicinal Effects |
Extraction and Isolation Method |
References |
|---|---|---|---|---|
|
Fucosylated chondroitin sulfate |
Body wall |
Antithrombotic, anticoagulant, anticancer, anti-inflammatory, antitumor, antidiabetic, anti-osteoarthritis, alleviates inflammation, alleviates pain, and improve immune system |
Enzymatic (papain/ Alcalase) hydrolysis followed by precipitation (cetylpyridinium chloride/ ethanol/ sodium hydroxide/ tricholoracetic acid) |
|
|
Collagen |
Body wall |
Antihypertension, antiaging, anti-wrinkle, alleviates skin problems, and wound healing |
A divalent cation chelator (EDTA) followed by extraction in water |
|
|
glycosides (saponins) |
Body wall |
Antibacterial, antifungal, antiviral, antitumor, anticancer, antiangiogenic, and photo-protective |
Isopropyl alcohol/ water extraction and refluxing with chloroform/ methanol/ethanol |
|
|
Fucoidan |
Body wall |
Anticoagulant, antibacterial, antiaging, anti-hyperglycemic, lowering blood glucose level, and photo-protective |
Hydrolysis with papain and precipitation with cetylpyridinium chloride |
|
|
Phenolic compounds |
Body wall, tentacles, and viscera |
Antioxidants and antibacterial |
Solvent extraction (methanol), water, organic solvent (ethyl acetate) and a mixture of water/ miscible organic solvent (acetonitrile) |
|
|
Cerebrosides |
Body wall |
Anticancer, anti-inflammatory, and anti-adipogenic activity |
Solvent extraction (65% ethanol) and isolated by High-performance liquid chromatography (HPLC), extracted by chloroform/ methanol using high speed counter-current chromatography |
|
|
Amino acid |
Body wall, tentacles, and viscera |
Anti-fatigue, repairing tissue, nutritional storage, and wound healing |
Reversed phase HPLC |
|
|
Protein (bioactive peptide) |
Body wall |
Antimicrobial |
Fractionated utilizing ammonium sulfate precipitation and analyzed by size exclusion chromatography |
[32] |
|
Vitamin and minerals |
Body wall, tentacles, and viscera |
Cosmeceutical properties, promote healthy growth and metabolism, lower the blood sugar level |
Association of Official Analytical Chemists (AOAC)-and inductively coupled plasma mass spectrometry (ICP-MS) |
|
|
Omega-3 (EPA) |
Body wall, tentacles, and viscera |
Anti-hyperglycemic, decrease cholesterol, and protect the heart |
Solvent extraction (methanol: chloroform: water) and analyzed by gas chromatography (GC)/ HPLC |
References
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474.
- Eckert, G.L. Sea cucumbers. In Encyclopedia of Tidepools and Rocky Shores; Denny, M.W., Gaines, S.D., Eds.; University of California Press: London, UK, 2007; pp. 491–492.
- Purcell, S.W.; Lovatelli, A.; Vasconcellos, M.; Ye, Y. Managing Sea Cucumber Fisheries with an Ecosystem Approach; FAO: Rome, Italy, 2010.
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223.
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods -A review. Mar. Drugs 2011, 9, 1761–1805.
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Z.Q.; Liu, Y.F.; Song, L.; Dong, X.P.; Li, D.M.; Zhu, B.W.; Konno, K.; et al. Effects of endogenous cysteine proteinases on structures of collagen fibres from dermis of sea cucumber (Stichopus japonicus). Food Chem. 2017, 232, 10–18.
- Trotter, J.A.; Lyons-Levy, G.; Chino, K.; Koob, T.J.; Keene, D.R.; Atkinson, M.A.L. Collagen fibril aggregation-inhibitor from sea cucumber dermis. Matrix Biol. 1999, 18, 569–578.
- Zhou, X.; Zhou, D.-Y.; Yin, F.-W.; Song, L.; Liu, Y.-X.; Xie, H.-K.; Gang, K.-Q.; Zhu, B.-W.; Shahidi, F. Glycerophospholipids in sea cucumber (Stichopus japonicus) and its processing by-products serve as bioactives and functional food ingredients. J. Food Bioact. 2018, 1, 134–142.
- Aminin, D.L.; Chaykina, E.L.; Agafonova, I.G.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A. Antitumor activity of the immunomodulatory lead Cumaside. Int. Immunopharmacol. 2010, 10, 648–654.
- Nagase, B.H.; Enjyoji, K.; Minamiguchi, K.; Kitazato, K.T.; Kitazato, K.; Saito, H.; Kato, H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: Antithrombin 111- and heparin cofactor 11-independent inhibition of factor x activation by factor ixa-factor viiia complex and heparin cofactor II-dependent inhibition of thrombin. Blood 1995, 1527–1534.
- Vieira, R.P.; Mulloy, B.; Mourão, P.P. Structure of a fucose-branched chondroitin sulfate from sea cucumber. J. Biol. Chem. 1991, 266, 13530–13536.
- Mourao, P.A.S.; Bastos, G.I. Highly acidic glycans from sea cucumbers. Eur. J. Biochem. 1987, 645, 639–645.
- Mourao, P.A.S.; Pereira, M.S.; Pavao, M.S.G. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. J. Biol. Chem. 1996, 271, 23973–23984.
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946.
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346.
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047.
- Ahmed, M.R.; Venkateshwarlu, U.; Jayakumar, R. Multilayered peptide incorporated collagen tubules for peripheral nerve repair. Biomaterials 2004, 25, 2585–2594.
- Mojica, E.R.E.; Merca, F. Lectin from the body walls of black sea cucumber (Holothuria atra Jäger). Philipp. J. Sci. 2004, 133, 77–85.
- Ming-Ping, L.A.; Jun-Jie, S.; Jian, J.; Yang-Hua, Y.I. Three cerebrosides from the sea cucumber Cucumaria frondosa. Chin. J. Nat. Med. 2012, 10, 105–109.
- Sugawara, T.; Zaima, N.; Yamamoto, A.; Sakai, S.; Noguchi, R.; Hirata, T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci. Biotechnol. Biochem. 2006, 70, 2906–2912.
- Findlay, J.A.; Daljeet, A.; Moharir, Y.E. Some constituents of the sea cucumber Cucumaria frondosa. Mar. Chem. 1983, 12, 228.
- Yahyavi, M.; Afkhami, M.; Javadi, A.; Ehsanpour, M.; Khazaali, A.; Khoshnood, R.; Mokhlesi, A. Fatty acid composition in two sea cucumber species, Holothuria scabra and Holothuria leucospilata from Qeshm Island (Persian Gulf). Afr. J. Biotechnol. 2012, 11, 2862–2868.
- Chen, J. Overview of sea cucumber farming and sea ranching practices in China. SPC Beche-mer Inf. 18 Bull. 2003, 18, 18–23.
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923.
- Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo anti-cancer mechanism of low-molecular-weight fucosylated chondroitin sulfate (LFCS) from sea cucumber Cucumaria frondosa. Molecules 2016, 21, 625.
- Tian, F.; Zhang, X.; Tong, Y.; Yi, Y.; Zhang, S.; Li, L.; Sun, P.; Lin, L.; Ding, J. PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 874–882.
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696.
- Mourão, P.A.S.; Boisson-Vidal, C.; Tapon-Bretaudière, J.; Drouet, B.; Bros, A.; Fischer, A.M. Inactivation of thrombin by a fucosylated chondroitin sulfate from echinoderm. Thromb. Res. 2001, 102, 167–176.
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.S.; Esko, J.D.; Pavão, M.S.G. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber: Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991.
- Whitehouse, M.W.; Fairlie, D.P. Anti-Inflammatory activity of a holothurian (sea cucumber) food supplement in rats. Inflammopharmacology 1994, 2, 411–417.
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636.
- Beauregard, K.A.; Truong, N.T.; Zhang, H.; Lin, W.; Beck, G. The detection and isolation of a novel antimicrobial peptide from the echinoderm, Cucumaria frondosa. Adv. Exp. Med. Biol. 2001, 484, 55–62.
- Hing, H.; Ambia, K.M.; Azraul-Mumtazah, R.; Hamidah, S.; Sahalan, A.; Shamsudin, N.; Shamsudin, M.; Hashim, R. Effect of methanol extracts from sea cucumbers Holothuria edulis and Stichopus chloronotus on Candida albicans. Microsc. Microanal. 2007, 13, 48–50.
- Althunibat, O.Y.; Hashim, R.B.; Taher, M.; Daud, J.M.; Ikeda, M.-A.; Zali, B.I. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009, 37, 376–387.
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016, 51, 650–658.
- San Miguel-Ruiz, J.E.; García-Arrarás, J.E. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev. Biol. 2007, 7, 115.
- Dong, X.; Pan, R.; Deng, X.; Chen, Y.; Zhao, G.; Wang, C. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2014, 49, 1352–1361.
- Siahaan, E.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals properties of sea cucumbers: Prospects and trends. Cosmetics 2017, 4, 26.
- Taiyeb-Ali, T.B.; Zainuddin, S.L.A.; Swaminathan, D.; Yaacob, H. Efficacy of “Gamadent” toothpaste on the healing of gingival tissues: A preliminary report. J. Oral Sci. 2003, 45, 153–159.
- Fredalina, B.D.; Ridzwan, B.H.; Abidin, A.A.Z.; Kaswandi, M.A.; Zaiton, H.; Zali, I.; Kittakoop, P.; Mat Jais, A.M. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen. Pharmacol. 1999, 33, 337–340.
- Hamel, J.F.; Mercier, A. Diet and feeding behaviour of the sea cucumber Cucumaria frondosa in the St. Lawrence estuary, eastern Canada. Can. J. Zool. 1998, 76, 1194–1198.
- Pantin, J.R.; Coughlan, E.J.; Hynick, E.M.; Skanes, K.R. An assessment of the sea cucumber (Cucumaria frondosa) resource on the St. Pierre Bank (NAFO subdivision 3Ps) in 2016. DFO Can. Sci. Advis. Secr. 2018, 024, 23.
- Koob, T.J.; Koob-Emunds, M.M.; Trotter, J.A. Cell-derived stiffening and plasticizing factors in sea cucumber (Cucumaria frondosa) dermis. J. Exp. Biol. 1999, 202, 2291–2301.
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale. Proc. Natl. Acad. Sci. USA 2016, 113, E6362–E6371.
- DFO. An assessment of the Sea Cucumber (Cucumaria frondosa) Resource on the St. Pierre Bank (NAFO subdivision 3PS) in 2016. Newfoundland and Labrador Region, Science Advisory Report; Fisheries and Oceans, Science, Newfoundland and Labrador Region: St. John’s, NL, Canada, 2017; pp. 1–8.
- Hamel, J.; Mercier, A. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can. J. Fish. Aquat. Sci. 1996, 53, 253–271.
- Bruckner, A.W. The recent status of sea cucumber fisheries in the continental United States of America. SPC Beche-mer Inf. Bull. 2005, 22, 39–46.
- Hamel, J.-F.; Mercier, A. Precautionary Management of Cucumaria frondosa in Newfoundland and Labrador, Canada. In A Global Review of Fisheries and Trade, FAO Fisheries and Aquaculture Technical Paper, No. 516; FAO: Rome, Italy, 2008; pp. 293–306.
- Nelson, E.J.; MacDonald, B.A.; Robinson, S.M.C. A review of the northern sea cucumber Cucumaria frondosa (Gunnerus, 1767) as a potential aquaculture species. Rev. Fish. Sci. 2012, 20, 212–219.
- Gianasi, B.L.; Parrish, C.C.; Hamel, J.-F.; Mercier, A. Influence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber Cucumaria frondosa. Aquac. Res. 2017, 48, 3413–3432.
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional Characteristics and Antioxidant Properties of Fresh and Processed Sea Cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 1188–1192.
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Proximate composition and nutritional profile of by-products from green urchin and Atlantic sea cucumber processing plants. Int. J. Food Sci. Technol. 2010, 45, 2119–2126.
- Kale, V.; Freysdottir, J.; Paulsen, B.S.; Friojónsson, Ó.H.; Óli Hreggviosson, G.; Omarsdottir, S. Sulphated polysaccharide from the sea cucumber Cucumaria frondosa affect maturation of human dendritic cells and their activation of allogeneic CD4(+) T cells in vitro. Bioact. Carbohydrates Diet. Fibre 2013, 2, 108–117.
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12.
- Hu, S.; Li, S.; Song, W.; Ji, L.; Cai, L.; Wang, Y.; Jiang, W. Fucoidan from Cucumaria frondosa inhibits pancreatic islets apoptosi through mitochondrial signaling pathway in insulin resistant mice. Food Sci. Technol. Res. 2016, 22, 507–517.
- Malavaki, C.; Mizumoto, S.; Karamanos, N.; Sugahara, K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008, 49, 133–139.
- Shi, Y.; Meng, Y.-C.; Li, J.; Chen, J.; Liu, Y.; Bai, X. Chondroitin sulfate: Extraction, purification, microbial and chemical synthesis. J. Chem. Technol. Biotechnol. 2014, 89, 1445–1465.
- Kim, C.-T.; Gujral, N.; Ganguly, A.; Suh, J.-W.; Sunwoo, H.H. Chondroitin sulphate extracted from antler cartilage using high hydrostatic pressure and enzymatic hydrolysis. Biotechnol. Rep. 2014, 4, 14–20.
- Santos, M.A.C.; Barcellos, P.G.; Queiroz, I.N.L.; Santos, G.R.C.; Soares, P.A.G.; Pomin, V.H.; Mourao, P.A.S.; Ribeiro, K.A.; Pereira, M.S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber using hydrogen peroxide and copper ions. In Proceedings of the 44th Annual Meeting of the Brazilian Society for Biochemistry and Molecular Biology, Foz do Iguacu, Brazil, 24–28 August 2015.
- Srichamroen, A.; Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Chondroitin sulfate extraction from broiler chicken cartilage by tissue autolysis. LWT Food Sci. Technol. 2013, 50, 607–612.
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614.
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-β-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596.
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82.
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 989–1000.
- Kariya, Y.; Mulloy, B.; Imai, K.; Tominaga, A.; Kaneko, T.; Asari, A.; Suzuki, K.; Masuda, H.; Kyogashima, M.; Ishii, T. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydr. Res. 2004, 339, 1339–1346.
- Wang, Y.; Su, W.; Zhang, C.; Xue, C.; Chang, Y.; Wu, X.; Tang, Q.; Wang, J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012, 133, 1414–1419.
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K/PKB pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42.
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50.
- Dave, D.; Liu, Y.; Clark, L.; Dave, N.; Trenholm, S.; Westcott, J. Availability of marine collagen from Newfoundland fisheries and aquaculture waste resources. Bioresour. Technol. Rep. 2019, 7, 100271.
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557.
- Wood, A.; Ogawa, M.; Portier, R.J.; Schexnayder, M.; Shirley, M.; Losso, J.N. Biochemical properties of alligator (Alligator mississippiensis) bone collagen. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 246–249.
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the major edible component of sea cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322.
- Liu, Y.X.; Liu, Z.Q.; Song, L.; Ma, Q.R.; Zhou, D.Y.; Zhu, B.W.; Shahidi, F. Effects of collagenase type I on the structural features of collagen fibres from sea cucumber (Stichopus japonicus) body wall. Food Chem. 2019, 301, 125302.
- Tipper, J.P.; Lyons-Levy, G.; Atkinson, M.A.L.; Trotter, J.A. Purification, characterization and cloning of tensilin, the collagen-fibril binding and tissue-stiffening factor from Cucumaria frondosa dermis. Matrix Biol. 2002, 21, 625–635.
- Trotter, J.A.; Lyons-Levy, G.; Thurmond, F.A.; Koob, T.J. Covalent composition of collagen fibrils from the dermis of the sea cucumber, Cucumaria frondosa, a tissue with mutable mechanical properties. Comp. Biochem. Physiol. Part Physiol. 1995, 112, 463–478.
- Nigrelli, R.F.; Jakowska, S. Effects of Holothurin, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann. N. Y. Acad. Sci. 1960, 90, 884–892.
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A.; Islam, M.T. Sea cucumber glycosides: Chemical structures, producing species and important biological properties. Mar. Drugs 2017, 15, 317.
- Avilov, S.A.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Gudimova, E.N.; Riguera, R.; Jimenez, C.; Frondoside, C. A new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: Structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 1998, 76, 137–141.
- Findlay, J.A.; Daljeet, A.; Matsoukas, J.; Moharir, Y.E. Constituents of the sea cucumber cucumaria frondosa. J. Nat. Prod. 1984, 47, 560.
- Findlay, J.A.; Yayli, N.; Radics, L. Novel sulfated oligosaccharides from the sea cucumber cucumaria frondosa. J. Nat. Prod. 1992, 55, 93–101.
- Yayli, N. Minor saponins from the sea cucumber Cucumaria frondosa. Indian J. Chem. Sect. B Org. Med. Chem. 2001, 40, 399–404.
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236.
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.-X.; Harvey, C.; Brisson, J.-R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990, 68, 11–18.
- Yayli, N.; Findlay, J.A. A triterpenoid saponin from Cucumaria frondosa. Phytochemistry 1999, 50, 135–138.
- Tian, F.; Zhu, C.; Zhang, X.; Xie, X.; Xin, X.; Yi, Y.; Lin, L.; Geng, M.; Ding, J. Philinopside E, A new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and avb3 integrin via binding to the extracellular domain of KDR. Mol. Pharmacol. 2007, 72, 545–552.
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990; pp. 101–126.
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184.
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2009, 45, 147–154.
- Stefaniak-Vidarsson, M.M.; Kale, V.A.; Gudjónsdóttir, M.; Marteinsdottir, G.; Fridjonsson, O.; Hreggvidsson, G.O.; Sigurjonsson, O.E.; Omarsdottir, S.; Kristbergsson, K. Bioactive effect of sulphated polysaccharides derived from orange-footed sea cucumber (Cucumaria frondosa) toward THP-1 macrophages. Bioact. Carbohydr. Diet. Fibre 2017, 12, 14–19.
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016, 25, 940–952.
- Mamelona, J.; Pelletier, E. Producing high antioxidant activity extracts from echinoderm by products by using pressured liquid extraction. Biotechnology 2010, 9, 523–528.
- Hossain, A.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant activity of Atlantic sea cucumber (Cucumaria frondosa). In Proceedings of the International Society for Nutraceuticals and Functional Foods (ISNFF), Vancouver, BC, Canada, 14–17 October 2018.
- Xu, C.; Zhang, R.; Wen, Z. Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 2018, 49, 73–84.
- Liu, X.; Hao, J.; Shan, X.; Zhang, X.; Zhao, X.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Yu, G. Antithrombotic activities of fucosylated chondroitin sulfates and their depolymerized fragments from two sea cucumbers. Carbohydr. Polym. 2016, 152, 343–350.
- Aminin, D.L.; Agafonova, I.G.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Collin, P.D.; Woodward, C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food 2008, 11, 443–453.
- Al Shemaili, J.; Mensah-Brown, E.; Parekh, K.; Thomas, S.A.; Attoub, S.; Hellman, B.; Nyberg, F.; Adem, A.; Collin, P.; Adrian, T.E. Frondoside A enhances the antiproliferative effects of gemcitabine in pancreatic cancer. Eur. J. Cancer 2014, 50, 1391–1398.
- Xu, J.; Guo, S.; Du, L.; Wang, Y.M.; Sugawara, T.; Hirata, T.; Xue, C.H. Isolation of cytotoxic glucoerebrosides and long-chain bases from sea cucumber Cucumaria frondosa using high speed counter-current chromatography. J. Oleo Sci. 2013, 62, 133–142.
- Xu, H.; Wang, F.; Wang, J.; Xu, J.; Wang, Y.; Xue, C. The WNT/β-catenin pathway is involved in the anti-adipogenic activity of cerebrosides from the sea cucumber Cucumaria frondosa. Food Funct. 2015, 6, 2396–2404.
- Hu, S.; Xu, L.; Shi, D.; Wang, J.; Wang, Y.; Lou, Q.; Xue, C. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. J. Biosci. Bioeng. 2014, 117, 457–463.




