Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisa Mazza | + 1954 word(s) | 1954 | 2021-12-29 03:52:03 | | | |
| 2 | Rita Xu | Meta information modification | 1954 | 2022-01-13 02:40:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mazza, E. Mediterranean Diet and COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/18131 (accessed on 07 February 2026).
Mazza E. Mediterranean Diet and COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/18131. Accessed February 07, 2026.
Mazza, Elisa. "Mediterranean Diet and COVID-19" Encyclopedia, https://encyclopedia.pub/entry/18131 (accessed February 07, 2026).
Mazza, E. (2022, January 12). Mediterranean Diet and COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/18131
Mazza, Elisa. "Mediterranean Diet and COVID-19." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Mediterranean Diet represents the traditional eating habits of populations living around the Mediterranean Sea, and it is associated with a lower risk of overall mortality and cancer incidence and cardiovascular diseases. Severe acute respiratory syndrome coronavirus 2 is a new pandemic, and represents a significant and critical threat to global human health.
Mediterranean diet
COVID-19
inflammation
nutrition
1. Introduction
The first part of 2020 was characterized by the pandemic spread of a novel coronavirus: severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. To date, millions of cases of coronavirus disease 19 (COVID-19) have been reported, and it has caused more than 3.9 million deaths in the world [2]. The population generally is not provided with immunity to SARS-CoV-2 and is susceptible to the new virus disease [1][3][4][5]. Previous epidemiological and clinical studies featuring COVID-19 have shown that SARS-CoV-2 infection usually results in mild disease, although several patients occasionally develop severe or critical illness [1][3][4][5][6][7][8]. In particular, asymptomatic individuals are estimated to range from 17.9% to 78% [3], approximately 15% of infected individuals will develop severe illness, and about 5% will eventually develop severe pneumonia and acute respiratory distress syndrome (ARDS) [4]. Some COVID-19 patients will also develop systemic manifestations such as sepsis, cardiovascular complications, thromboembolism, and multi-organ failure [4][8].
Worsening clinical outcomes of COVID-19 have been associated with older age, male gender, and the presence of comorbidities such as hypertension, obesity, and type 2 diabetes mellitus [5][6][7].
A recent report shows data from the COVID-19 case fatality rate (CFR) in Italy, highlighting a linear relationship between the CFR and age [9]. In particular, CFRs are less than 0.4% in patients aged 40 or younger, 1% among those aged 50, 3.5% in those aged 60, 12.8% in those aged seventy, at 20.2% in those over eighty; the overall CFR is 7.2% [9]. Recent evidence suggests that the SARS-CoV-2 viral loads are similar in asymptomatic, mild symptomatic, and severe symptomatic patients [10][11], but many other factors influence the progression and severity of the disease.
Currently, no specific data have been reported regarding the immunological response to SARS-CoV-2, but new studies have shown that a cytokine storm overstimulates the body’s immune response to microorganisms as a consequence of increases in the levels of inflammatory factors [12]. Therefore, the inflammatory factors contribute to one of the most important mechanisms underlying disease progression and death. Then, the coexistence of both COVID-19 and chronic diseases should be considered alarming, because it represents the combination of more pandemics [13]. The interaction between nutrition, immune function, inflammation, and infection represents a key tool to reduce the risk of susceptibility and morbidity of viral infectious diseases [13][14][15]. Research has shown that greater adherence to the Mediterranean diet (MetDiet) is associated with a reduced risk of major chronic diseases, [16] due to its anti-inflammatory and immune-modulatory properties.
2. The Mediterranean Diet: A Healthy Dietary Pattern for People with SARS-CoV-2 Infection
The MetDiet is a model of eating based on the traditional foods and drinks of the countries surrounding the Mediterranean Sea. Over the last few decades, this nutritional model has been promoted worldwide as one of the healthiest dietary patterns and has been reported to be consistently beneficial with regard to longevity. The MetDiet is characterized by high consumption of unrefined cereals, fruit, vegetables, legumes, and olive oil, moderate consumption of dairy products and wine, and low meat consumption [16][17].
Among other benefits, adhering to the MetDiet has been linked to a lower risk of various chronic conditions [18][19][20][21][22], with lower risk of inflammation as well as increased immunity [23][24]. Its protective properties are thought to be a combination of the high intake of polyunsaturated fatty acids (PUFA) from fish [25], monounsaturated fatty acids (MUFA) and polyphenols from extra virgin olive oil (EVOO) [26], and antioxidants from fruit, vegetables, legumes, and wine [20][26][27]. Furthermore, the MetDiet is rich in phytochemicals with antioxidant action, minerals, and vitamins [23].
The first umbrella review meta-analysis of observational studies and randomized trials estimated the association between adherence to the MetDiet and 37 different health outcomes, including overall mortality, cardiovascular and cancer outcomes, neurodegenerative and metabolic disorders, as well as inflammatory markers. This meta-analysis showed that a greater adherence to the MetDiet reduced the risk of overall mortality and cancer incidence, cardiovascular and neurodegenerative diseases, and diabetes [16].
Each component of the MetDiet has its benefits, but it can be assumed that it is the combination of various nutrients that is the basis of the extraordinary health effects of MetDiet [16][17], especially on the immune system [28][29].
Recent research showed that one MetDiet-style meal reduced the expression of pro-inflammatory molecules [29], the overall systemic inflammatory status [30], and several diseases associated with chronic low-grade inflammation. In adult individuals, a MetDiet intervention led to lower glycoxidative impairment [31] and inflammatory response [32][33]. A meta-analysis including 2300 subjects reported a significant reduction in high-sensitivity C-reactive protein (hs-CRP) (−0.98 mg/L, p < 0.0001), intracellular adhesion molecule-1 (−23.73 ng/mL, p = 0.008), and IL-6 (−0.42 pg/mL, p = 0.008) in individuals assigned to MetDiet, compared with those following a control intervention protocol [34].
A potential protection against COVID-19 by a MetDiet was assessed longitudinally in a cohort of 5194 non-health professionals [35]. Participants with the highest adherence to MetDiet had a significantly lower odds of developing SARS-CoV-2 infection compared with those with lowest adherence (multivariable-adjusted OR = 0.36, 95% CI: 0.16–0.84; p for trend < 0.001) [35].
An ecological study, of only European countries, showed a significant negative association between MetDiet and COVID-19-related deaths (r2 = 0.771, p = 0.030) [36]. The authors observed that MetDiet adherence was negatively associated with COVID-19 cases as well as related deaths across 17 regions in Spain and that the relationship remained also after adjustment for factors of well-being [36]. The same authors also observed a negative association between Metdiet adherence and COVID-19-related deaths across 23 countries (OECD) after adjustment for physical inactivity and some confounding factors [36].
An observational case control study explored the possible associations among different dietary patterns and COVID-19 events and outcomes. The results showed that the cases had a lower mean of the MedDiet score (p = 0.009) than controls did, demonstrating an inverse association between the MetDiet and COVID-19 risk [37].
The preliminary results of an experimental study aimed to detect the beneficial effects of MetDiet before and after the period of COVID-19 Lockdown in Mediterranean area (Spain) old individuals showed that patients who initiated the MetDiet intervention program before Lockdown increased their level of adherence to the MetDiet by 3.5% and maintained an adequate nutritional status after the Lockdown. In the BMI, there no were statistically significant differences between experimental and control groups before and after Lockdown. These results suggest that adherence to the MetDiet may play an important role in the maintenance of an adequate nutritional status in the confinement situations such as the COVID-19 Lockdown [38].
All these results suggest the important role that nutrition, and, in particular, the MetDiet, could play in the prevention and management of COVID-19 infection (Figure 1).
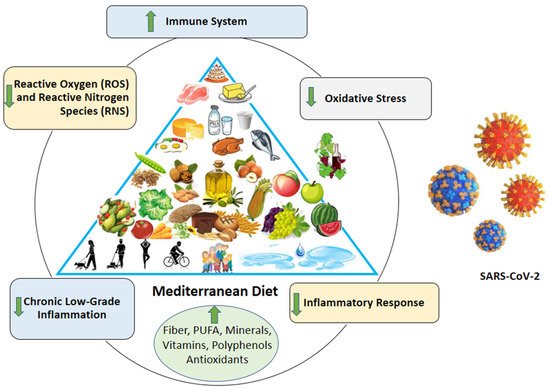
Figure 1. Mediterranean diet: potential strategy against coronavirus infection.
3. Mediterranean Diet and COVID-19: Plausible Mechanisms of Potential Benefits
COVID-19 is characterized by increased levels of numerous cytokines, mainly of proinflammatory character, including tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and CRP [39]. Therefore, effective treatment strategies pursued could include reducing inflammation in order to prevent the risk of infection or blunt the severity of the COVID-19 disease [12]. In this regard, several studies suggest that MetDiet induces positive effects on both inflammation and oxidative stress. The stimulating effect induced at the level of the immune system is pointed out by the positive results induced by MetDiet on people with inflammatory phenomena impacting the respiratory system [40]. Several micronutrients have been suggested to act as immunomodulatory agents against COVID-19, and they are briefly summarized in Table 1.
Table 1. Possible anti-SARS-CoV-2 effects attributed to MetDiet.
| Effects | Components | Food Sources | References |
|---|---|---|---|
| Lower Inflammation (CRP, IL-6, TNF-alpha, ROS, RNS) | PUFA, MUFA, polyphenols, antioxidants, fibers, vitamins, minerals | Fish, EVOO, fruit, vegetables, legumes, wine, whole grains | [16][20][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70] |
| Boost Immune system (anti-thrombotic, anti-PAF effect) and antiviral effects (NF-κB, AP-1) | Vitamin A, C, E, D, selenium, zinc, phytochemicals, and omega-3 PUFA, polifenols, antioxidants, resveratrol, | Legumes, vegetables, fruit, EVOO, seeds, bran, nuts and dried fruit, shellfish, beef, tea, red wine | [14][42][48][51][52][53][57][58][59][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88] |
| Boost Intestinal Barrier Function (gut microbiota) | Prebiotic substances, galactans, fructans, fibers, and inulins | Legumes, vegetables, fruit, nuts, seeds, bran, milk and yogurt | [46][47][48][64][65][72][89][90][91][92][93][94][95] |
| Improvement of the metabolic setting (ACE2, Leptin) | PUFA, MUFA, polyphenols, antioxidants, fibers, vitamins, minerals, prebiotic substances, polifenols, antioxidants, resveratrol | Legumes, vegetables, fruit, EVOO, seeds, bran, nuts and dried fruit, shellfish, beef, tea, red wine | [13][14][15][16][17][18][19][20][21][22][96][97][98][99][100][101] |
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; TNF-alpha, tumor necrosis factor-alpha; ROS, reactive oxygen species; RNS, reactive nitrogen species; PUFA, polyunsaturated fatty acids; EVOO, extra virgin olive oil.
Fruits, whole grains, vegetables, fish, PUFA, and MUFA have been found to cause less inflammation in the body [41], while foods with high saturated fat content such as processed red meat, cheese, and dairy may induce inflammation [40]. It may be the abundance of beneficial foods (rich in fiber, PUFA, minerals, vitamins, polyphenols, and antioxidants) and lack of fatty foods (rich in starch, refined sugar and trans fatty acids) in the MetDiet that produce its favorable effects [42].
PUFAs include long-chain omega-3 PUFAs, EPA (20:5n–3), and DHA (22:6n–3), derived mainly from fish and seafood [41], as well as α-linolenic acid, derived from various plant sources [43]. Among PUFAs, the omega-3 free fatty acids exert anti-inflammatory effects via specialized pro-resolving mediators, which are the oxylipins, of oxygenated metabolites [25][44].
Dietary fibers are an important factor regarding the influence of complex carbohydrates on inflammation [45][46]. It was demonstrated that an increase in fiber consumption (about 30 g/d) was associated with a significant reduction in hs-CRP concentrations [47]. Another advantage of dietary fiber intake is a more favorable gut microbiome composition, which lowers both gut and systemic inflammation, and even small increases of fiber (5 g/d) can be beneficial [89][90]. Watanabe et al. hypothesized that a rice-eating habit seems to be a factor that explains the reason for low COVID-19 incidence and mortality in rice-eating countries. The authors make a hypothesis that populations who consume rice have a special profile of microbiota that produce butyrate, which stimulates the proliferation of regulatory T cells, prevents a cytokine storm (induced by the infection), and reduces the levels of IL-6 and CRP [91].
Although it is the most consumed food in Asia, rice plays a key role also in the diet of many countries, including those of the Mediterranean area [92].
Modifications in the intestinal barrier contribute to the pathogenesis of many illnesses; viruses may also contribute in disrupting the intestinal epithelium [93]. Sharma clarified that the gastrointestinal structure and the gut barrier may be affected by SARS- CoV-2 virus, and disorder of barrier functions or intestinal microbial dysbiosis may influence the progression and severity of COVID-19 disease [93]. It has been shown that the SARS-CoV-2 virus can impact PALS1, a tight junction-associated protein, present in the intestinal and lung epithelium [71]. For this, it has been proposed that SARS-CoV-2 may increase intestinal permeability, causing damage to enterocytes and the epithelial layer [72].
MetDiet is also very rich in prebiotic substances, such as galactans, fructans, fibers, and inulins. Numerous reports indicate that these compounds are used by host microorganisms, supporting the growth of favorable bacteria and by promoting the production of beneficial metabolites [48][49][93].
There is also evidence supporting the protective role of vitamins against viral infections through multiple mechanisms [44]. EVOO is one of the staple foods of the MetDiet, and is the main dietary source of vitamin E. This vitamin is one of the most effective nutrients enhancing immune function and inflammation [44][50]. Several studies have indicated that vitamin E deficiency impairs both humoral and cell-mediated immune functions [51][52]. Vitamin E and vitamin C are well-known antioxidant compounds, able to reduce the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [44][73]. Moreover, Vitamin A is involved in the production of mucin secretion and enhancing antigen nonspecific immunity functions (healthy mucus stratum), such as those of the bowel and the respiratory tract [51][52].
Many studies have highlighted the ability of vitamin D to reduce infections and to modulate innate and adaptive cellular immunity, and have shown an inverse association between the incidence of airway infections and its serum levels [74].
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059.
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3.
- Hu, Y.; Sun, J.; Dai, Z.; Deng, H.; Li, X.; Huang, Q.; Wu, Y.; Sun, L.; Xu, Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 127, 104371.
- Yang, R.; Gui, X.; Xiong, Y. Comparison of Clinical Characteristics of Patients with Asymptomatic vs. Symptomatic Coronavirus Disease 2019 in Wuhan, China. JAMA Netw. Open 2020, 3, e2010182.
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776.
- Wan, S.X.; Yi, Q.J.; Fan, S.B.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020.
- He, X.; Lau, E.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675.
- Kam, K.Q.; Yung, C.F.; Cui, L.; Tzer Pin Lin, R.; Mak, T.M.; Maiwald, M.; Li, J.; Chong, C.Y.; Nadua, K.; Tan, N.; et al. A Well Infant with Coronavirus Disease 2019 With High Viral Load. Clin. Infect. Dis. 2020, 71, 847–849.
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287.
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91–129.
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309.
- Keusch, G.T. The history of nutrition: Malnutrition, infection and immunity. J. Nutr. 2003, 133, 336S–340S.
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43.
- Maillot, M.; Issa, C.; Vieux, F.; Lairon, D.; Darmon, N. The shortest way to reach nutritional goals is to adopt Mediterranean food choices: Evidence from computer-generated personalized diets. Am. J. Clin. Nutr. 2011, 94, 1127–1137.
- Wallace, D.C. Mitochondrial genetics: A paradigm for aging and degenerative diseases? Science 1992, 256, 628–632.
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Foundation Expert Group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284.
- Mazza, E.; Fava, A.; Ferro, Y.; Moraca, M.; Rotundo, S.; Colica, C.; Provenzano, F.; Terracciano, R.; Greco, M.; Foti, D.; et al. Impact of legumes and plant proteins consumption on cognitive performances in the elderly. J. Transl. Med. 2017, 15, 109.
- Ferro, Y.; Mazza, E.; Salvati, M.; Santariga, E.; Giampà, S.; Spagnuolo, R.; Doldo, P.; Pujia, R.; Coppola, A.; Gazzaruso, C.; et al. Effects of a Portfolio-Mediterranean Diet and a Mediterranean Diet with or without a Sterol-Enriched Yogurt in Individuals with Hypercholesterolemia. Endocrinol. Metab. 2020, 35, 298–307.
- Colica, C.; Mazza, E.; Ferro, Y.; Fava, A.; De Bonis, D.; Greco, M.; Foti, D.P.; Gulletta, E.; Romeo, S.; Pujia, A.; et al. Dietary Patterns and Fractures Risk in the Elderly. Front. Endocrinol. 2017, 8, 344.
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227.
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148.
- Mazza, E.; Ferro, Y.; Lamprinoudi, T.; Gazzaruso, C.; Doldo, P.; Pujia, A.; Montalcini, T. Relationship between high sodium and low PUFA intake and carotid atherosclerosis in elderly women. Exp. Gerontol. 2018, 108, 256–261.
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the replacement of dietary vegetable oils with a low dose of extravirgin olive oil in the Mediterranean Diet on cognitive functions in the elderly. J. Transl. Med. 2018, 16, 10.
- DeKoning, L.; Anand, S.S. Adherence to a Mediterranean diet and survival in a Greek population. Trichopoulou A, Costacou T, Bamia C, Trichopoulos, D. N. Engl. J. Med. 2003, 348, 2599–2608. Vasc. Med. 2004, 9, 145–146.
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2018, 73, 318–326.
- De Lorenzo, A.; Bernardini, S.; Gualtieri, P.; Cabibbo, A.; Perrone, M.A.; Giambini, I.; Di Renzo, L. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: A randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2017, 54, 141–149.
- Bédard, A.; Lamarche, B.; Corneau, L.; Dodin, S.; Lemieux, S. Sex differences in the impact of the Mediterranean diet on systemic inflammation. Nutr. J. 2015, 14, 46.
- Lopez-Moreno, J.; Quintana-Navarro, G.M.; Delgado-Lista, J.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Camargo, A.; Perez-Martinez, P.; Tinahones, F.J.; Striker, G.E.; et al. Mediterranean Diet Supplemented with Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 340–346.
- Bordoni, L.; Petracci, I.; Zhao, F.; Min, W.; Pierella, E.; Assmann, T.S.; Martinez, J.A.; Gabbianelli, R. Nutrigenomics of Dietary Lipids. Antioxidants 2021, 10, 994.
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants 2021, 10, 906.
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 929–939.
- Perez-Araluce, R.; Martinez-Gonzalez, M.A.; Fernández-Lázaro, C.I.; Bes-Rastrollo, M.; Gea, A.; Carlos, S. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin. Nutr. 2021.
- Greene, M.W.; Roberts, A.P.; Frugé, A.D. Negative Association Between Mediterranean Diet Adherence and COVID-19 Cases and Related Deaths in Spain and 23 OECD Countries: An Ecological Study. Front. Nutr. 2021, 8, 591964.
- El Khoury, C.N.; Julien, S.G. Inverse Association Between the Mediterranean Diet and COVID-19 Risk in Lebanon: A Case-Control Study. Front. Nutr. 2021, 8, 707359.
- Zaragoza-Martí, A.; Sánchez-SanSegundo, M.; Ferrer-Cascales, R.; Gabaldón-Bravo, E.M.; Laguna-Pérez, A.; Rumbo-Rodríguez, L. Effects of the Mediterranean Lifestyle During the COVID-19 Lockdown in Spain: Preliminary Study. Front. Nutr. 2021, 8, 683261.
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75.
- Margină, D.; Ungurianu, A.; Purdel, C.; Nițulescu, G.M.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Burykina, T.I.; Tekos, F.; Buha, A.; et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020, 143, 111558.
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34.
- Bellastella, G.; Scappaticcio, L.; Esposito, K.; Giugliano, D.; Maiorino, M.I. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res. Clin. Pract. 2018, 143, 389–397.
- Jiang, L.; Wang, J.; Xiong, K.; Xu, L.; Zhang, B.; Ma, A. Intake of Fish and Marine n-3 Polyunsaturated Fatty Acids and Risk of Cardiovascular Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 2342.
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562.
- Bo, S.; Ciccone, G.; Guidi, S.; Gambino, R.; Durazzo, M.; Gentile, L.; Cassader, M.; Cavallo-Perin, P.; Pagano, G. Diet or exercise: What is more effective in preventing or reducing metabolic alterations? Eur. J. Endocrinol. 2008, 159, 685–691.
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922.
- North, C.J.; Venter, C.S.; Jerling, J.C. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur. J. Clin. Nutr. 2009, 63, 921–933.
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290.
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature reviews. Gastroenterol. Hepatol. 2021, 18, 196–208.
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703.
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494.
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614.
- Skalny, A.V.; Aschner, M.; Tinkov, A.A. Zinc. Adv. Food Nutr. Res. 2021, 96, 251–310.
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764.
- Ashraf, M.A.; Nookala, V. Biochemistry of Platelet Activating Factor; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462.
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817.
- Gao, X.; Bermudez, O.I.; Tucker, K.L. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J. Nutr. 2004, 134, 913–918.
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534.
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxidative Med. Cell. Longev. 2016, 2016, 9346470.
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti-Infect. Ther. 2016, 14, 57–80.
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35.
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381.
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131.
- De Ligt, M.; Hesselink, M.; Jorgensen, J.; Hoebers, N.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411.
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives—A biochemical perspective. Food Chem. 2021, 334, 127508.
- Flores-Félix, J.D.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Consumption of Phenolic-Rich Food and Dietary Supplements as a Key Tool in SARS-CoV-19 Infection. Foods 2021, 10, 2084.
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952.
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902.
- Shinde, T.; Hansbro, P.M.; Sohal, S.S.; Dingle, P.; Eri, R.; Stanley, R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms 2020, 8, 921.
- Teoh, K.T.; Siu, Y.L.; Chan, W.L.; Schlüter, M.A.; Liu, C.J.; Peiris, J.S.; Bruzzone, R.; Margolis, B.; Nal, B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol. Biol. Cell 2010, 21, 3838–3852.
- Uzzan, M.; Corcos, O.; Martin, J.C.; Treton, X.; Bouhnik, Y. Why is SARS-CoV-2 infection more severe in obese men? The gut lymphatics—Lung axis hypothesis. Med. Hypotheses 2020, 144, 110023.
- Hartmann, M.S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Vitamin E as promising adjunct treatment option in the combat of infectious diseases caused by bacterial including multi-drug resistant pathogens—Results from a comprehensive literature survey. Eur. J. Microbiol. Immunol. 2020, 10, 193–201.
- Getachew, B.; Tizabi, Y. Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity. J. Med Virol. 2021, 93, 5285–5294.
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 749–754.
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2014, 28, 364–371.
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710.
- Alpert, P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017, 29, 199–202.
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248.
- Lin, W.; Zhang, J.; Xu, J.F.; Pi, J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284.
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101.
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The Influence of Nutritional Factors on Immunological Outcomes. Front. Immunol. 2021, 12, 665968.
- Giampieri, F.; Cianciosi, D.; Ansary, J.; Elexpuru-Zabaleta, M.; Forbes-Hernandez, T.Y.; Battino, M. Immunoinflammatory effects of dietary bioactive compounds. Adv. Food Nutr. Res. 2021, 95, 295–336.
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Tahir Nadeem, M.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229.
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414.
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781.
- González-Gallego, J.; Sánchez-Campos, S.; Tuñón, M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293.
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of Gut Microbiota Biotransformation on Dietary Tannins and Human Health Implications. Microorganisms 2021, 9, 965.
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655.
- Capurso, C. Whole-Grain Intake in the Mediterranean Diet and a Low Protein to Carbohydrates Ratio Can Help to Reduce Mortality from Cardiovascular Disease, Slow Down the Progression of Aging, and to Improve Lifespan: A Review. Nutrients 2021, 13, 2540.
- Watanabe, S.; Inuma, K.; Kikuchi, K.; Yamamoto, T. “X Factor” of Japanese to Suppress COVID-19 Mortality. Acta Sci. Nutr. Health 2021, 5, 34–36.
- Bresciani, A.; Pagani, M.A.; Marti, A. Rice: A Versatile Food at the Heart of the Mediterranean Diet. In Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine; Boukid, F., Ed.; Springer: Cham, Switzerland, 2021.
- Sharma, L.; Riva, A. Intestinal Barrier Function in Health and Disease-Any role of SARS-CoV-2? Microorganisms 2020, 8, 1744.
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375.
- Franco, M.N.; Galeano-Díaz, T.; López, O.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298.
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826.
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89.
- Maiorino, M.I.; Bellastella, G.; Longo, M.; Caruso, P.; Esposito, K. Mediterranean Diet and COVID-19: Hypothesizing Potential Benefits in People with Diabetes. Front. Endocrinol. 2020, 11, 574315.
- Stark, A.H.; Madar, Z. Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr. Rev. 2002, 60, 170–176.
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943.
More
Information
Subjects:
Health Care Sciences & Services
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
906
Revisions:
2 times
(View History)
Update Date:
13 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




