Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mujib Ullah | + 2479 word(s) | 2479 | 2022-01-10 07:05:39 | | | |
| 2 | Amina Yu | Meta information modification | 2479 | 2022-01-11 03:18:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ullah, M. Isolation and Purification of EVs. Encyclopedia. Available online: https://encyclopedia.pub/entry/18006 (accessed on 07 February 2026).
Ullah M. Isolation and Purification of EVs. Encyclopedia. Available at: https://encyclopedia.pub/entry/18006. Accessed February 07, 2026.
Ullah, Mujib. "Isolation and Purification of EVs" Encyclopedia, https://encyclopedia.pub/entry/18006 (accessed February 07, 2026).
Ullah, M. (2022, January 11). Isolation and Purification of EVs. In Encyclopedia. https://encyclopedia.pub/entry/18006
Ullah, Mujib. "Isolation and Purification of EVs." Encyclopedia. Web. 11 January, 2022.
Copy Citation
Extracellular vesicles are sacs that are secreted by almost all types of cells and are responsible for intracellular communication. They inherit their content and characteristics from their donor cells. Pathological and physiological characteristics of donor cells are reflected in the appearance of specific nucleotide and proteins (on the EV surface or in their content).
extracellular vesicles
stem cells
isolation and purification methods
clinical application
1. Different Methods for EV Isolation and Purification
In the past few decades, there has been considerable attention on using extracellular vesicles as biomarkers for various conditions and as drug delivery vehicles. One of the challenges encountered for wide application is choosing an optimum, efficient and reliable isolation method [1][2]. Filtration, ultracentrifugation and affinity separation are the most common isolation methods . To isolate well-purified and healthy extracellular vesicles, a suitable combination of isolation and purification methods is necessary, Figure 1 [3][4].
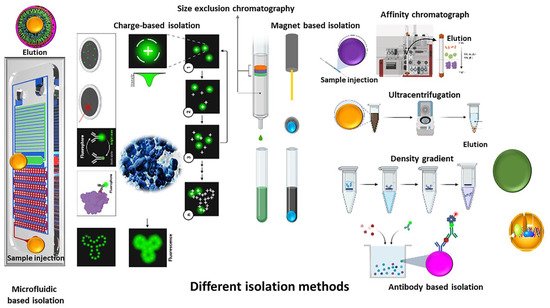
Figure 1. Schematic representation of different methods for extracellular isolation and purification.
Various peptides, proteins, lipids and cell debris contaminants are present in the source samples, some of which are similar to EVs in structure and composition, whereas some interact with EVs, preventing extraction [2][5][6].
In the following paragraphs, EV isolation methods are discussed in further detail, Figure 1.
1.1. Centrifugation-Ultracentrifugation-Density Gradient
A centrifuge is a device for separating particles from a solution according to their size, shape and density, and the viscosity of the medium. It causes denser elements (cells, particles, proteins) to separate at the bottom of a tube. The greater the difference in density, the faster they separate [7].
Ultracentrifugation is the gold standard method for extracellular vesicle isolation. The different types of ultracentrifugation are differential ultracentrifugation, density gradient centrifugation and rate-zonal centrifugation techniques [8].
Differential ultracentrifugation was the first technique that was used for extracellular vesicle isolation. This method is based on density, size and shape of the EVs [9][10][11][12]. The duration of centrifugation, temperature and sample dilution play pivotal role in separation efficiency [13][14]. It is easy to use and requires no or slight sample pretreatment, but it takes longer time, needs more labor and a large sample [15][16]. In addition, various types of EVs cannot be separated via this technique [12]. Separation via density gradient could be considered for reaching higher purification as described below .
Density gradient centrifugation (DGC) is another ultracentrifugation method. The difference between UC and DGC is, centrifugation occurs in a tube that contains preconstructed density gradient medium in case of DGC [10]. Sucrose and iodixanol (OptiPrep®) are the most used media. Through this technique, extracellular vesicles can be separated from proteins . Furthermore, various kinds of EVs could be separated based on their density [17]. Longer cycle durations, low yield rate and requirement of larger volume of sample (in comparison with UC) are the drawbacks of this technique [18][19].
Gradient centrifugation is rate-zonal centrifugation based on density gradient and sedimentation rate. The sample is added to the top of the tube and through centrifugation, compounds with higher density go through the dense layer, easier that lighter compounds. The duration of centrifugation should be controlled to avoid pellet construction at the bottom of the tube [20]. In addition, via this technique, particles with the same density and different diameter (size) can be separated [21]. This technique causes more extracellular recovery in comparison with density gradient centrifugation .
1.2. Precipitation
This method works based on dispersibility alteration . A water-excluding compound is first added to the sample. Polyethylene glycol (PEG), a polymer, is the commonly used compound for this purpose. After adding the polymer, centrifugation or filtration is needed for separation. The polymer dries the sample and leads to the precipitation of other molecules. [6][22].
Precipitation is considered a quick and easy method for EV isolation and can be used for small or large volume samples. In addition, this method requires little proficiency and not a specialized apparatus. The selectivity and quality of the isolate (unspecific co-precipitation) in this method is poor, and it must be combined with other method(s) [10][23][24]. To overcome this, filtration or ultracentrifugation can be carried out before treatment with PEG . In addition, some commercial precipitation kits have been developed [25]. These kits are fast, easy to use and do not require a specific apparatus. However, they are expensive, not applicable for large samples and are not efficient to separate different types of EVs [26][27]. Other compounds that can be used for precipitation are Acetat salt and protamine [26][28].
Precipitation should always be followed by centrifugation and filtration to eliminate contaminants [26][28].
Lectin is another chemical precipitant. In this technique, the sample is pretreated via centrifugation for separating cell debris and lectin is added to the sample and incubated overnight. Lectin conjugates with the carbohydrate of the exosome membrane, changes its solubility and causes precipitation, following which exosomes/EVs will be separated via centrifugation [29]. Chemical precipitations methods are simple, cost less, and are suitable for different sample sizes [26][28][30][31][32].
1.3. Size Based Approaches
As the title suggests, EV isolation here is based on size differentiation. Various techniques purify EVs based on size, including ultrafiltration, isolation kits, sequential filtration, size-exclusion chromatography (SEC), field-flow fractionation (FFF) and hydrostatic filtration dialysis (HFD) .
Ultrafiltration is the most common size-based isolation method. In this technique, sample goes through membrane filters with different pore sizes. EVs are then separated based on size and molecular weight . One of the limitations of ultrafiltration is EV clogging and trapping in the membrane. This can be prevented by initial filtration using large pore filters, followed by filtration through small pore filters [23][33]. The other drawbacks of ultrafiltration are poor efficiency and filter plugging [23][34][35]. Ultrafiltration also leads to deformation of EVs due to the pre membrane pressure (this disadvantage can be reduced by forcing lower pressure). However, the technique is still popular as it is less time-consuming and does not require expensive instruments [23][25][35].
In recent times, isolation kits based on size differentiation have been developed. One of them is the ExoMir Kit (Bioo Scientific; Austin, TX, USA) that contains two different membranes (upper membrane: 200 nm and downer: 20 nm) in a syringe .
In addition, ExoTIC (exosome total isolation chip) technology is the other kit that could purify EVs by passing the sample through different filters. These kits are easy to use, have a high yield rate and can be used for different types of bio-fluid samples [36][37]. Other methods are tangential flow filtration (TFF), direct filtration, and cyclic TFF [38].
Sequential filtration is another technique where the sample is passed through different filters. In each step particles with larger size than membrane pores are trapped, and smaller particles go through it. It is a semi-automated technique. Therefore, it is easy to use and less time-consuming [34]. Filter trapping is a limitation of sequential filtration that leads to membrane plugging and yield rate reduction [34][39][40].
Size-exclusion chromatography is another method that isolates EVs based on size. It consists of a column that allows penetration of smaller particles. This causes bigger particles to exit the column earlier. This protects the structure, integrity and biological function of EVs [34][41][42][43][44]. In this method, the sample does not rely on extensive pretreatment [34][42].
The first time that this method was developed, starch and water were used to form pores in the column, but through time, various other compounds such as dextran polymer (Sephadex), agarose (Sepharose) and polyacrylamide (Sephacryl or BioGel) have been used [34][45][46][47].
The other innovation for EV isolation based on the size differentiation is field-flow fractionation (FFF). In this method, sample is injected into a chamber that is affected via a cross flow, whereas bigger particles will be pushed to the walls of the chamber due to the cross flow, smaller particles elute earlier [48]. This method provides an opportunity to isolate various types of EVs and even very tiny compounds. It is faster, highly efficient, label-free and has higher sample recovery [49].
Another technique called hydrostatic dialysis isolation (HDI) uses hydrostatic forces for isolation. Small particles diffuse through the membrane and larger ones stay in the tube [50]. Via this method, the Tamm–Horsfall Protein, one of the abundant proteins in urinary EVs, is eliminated. After HDI, centrifugation is performed for further purification [51].
1.4. Affinity
Affinity based EV isolation is based on the antigens present on the EV surface. Antigens on the EV membrane are considered as markers to distinguish their sources [52][53][54]. These antigens are captured via specific antibodies [10][55]. This method provides highly purified EVs, but the harvest rate is low [24]. Pretreatment of samples, especially plasma, with ultracentrifugation or ultrafiltration is necessary [53]. In the study conducted by Tauro BJ et al., the efficacy and the results of three different methods including ultracentrifugation, density gradient isolation and immunoaffinity capture method indicate that immunoaffinity causes the highest purification of EV [52]. The limitation of this method is related to the availability of antibodies of the identified antigen. Masking of the antigens on the EV surface can prevent isolation via immunoaffinity capture methods [17]. Enzyme-linked immunosorbent assay (ELISA) is the most common immunoaffinity-based isolation and identification method [56]. Samples should be pretreated with ultracentrifugation before affinity capture .
One of the most effective methods to elevate EV harvest through immunoaffinity is increasing the surface area of presenting antibodies. Magneto-immunoprecipitation is a technique used for this. In this, a biotinylated antibody specific to the presenting antigen is attached to the surface of magnetic beads coated with streptavidin. Isolated EVs are then detached and used for other purposes while preserving the activity of EV protein [57]. This technique is easy and fast, but a high affinity between antigen and antibody can prevent the detachment of EVs [58].
In a study that was performed by Zhang J et.al, a combination of three methods was used to reach the optimum level of EV purification. Their protocol contains tangential flow filtration, centrifugation and immunomagnetic affinity technique; the first and second steps produce purified EVs and with immunomagnetic affinity; EVs that contain specific markers are isolated [59][60].
1.5. Micro-, Nano-Fluidics, Chips
Micro-, nano-fluidic chips isolate EVs based on their biochemical properties using acoustic, electrophoretic, and electromagnetic technology. This method is fast, inexpensive, efficient and can be used on small samples [61][62][63].
Microchips have been developed to isolate EVs with different approaches, including immunoaffinity, size and density-based separations. Through immunoaffinity capture, markers on the EV membrane bind to their specific antibody on the beads or inner surfaces modified by antibodies. The major limitation is the need for appearance of specific antigen on the EV surface. Developing size based microchips can surpass this limitation [63].
For size-based isolation of microchips, pressure and electrophoresis techniques are used. Electrophoresis is preferred in comparison with pressure as it prevents pore blockage [62][63].
In addition, nanowires, nano-sized deterministic lateral displacement (nano-DLD) and viscoelastic flow are the other techniques that can be used. The mechanism using nanowires is similar to SEC and contains micro-porous silicon. Nano-DLD is a pillar-array-based microfluidic method that categorizes elements in an incessant stream [63], whereas viscoelastic flow is a novel passive and label-free technique in this category that separate particles based on variance among elastic lift forces executed on compounds with different sizes in a viscoelastic medium [63][64].
Acoustic separation is one of the techniques that is used via micro-fluidic chips. In this technique, the sample is exposed to ultrasonic waves. The larger particles are affected via heavier radiation and transferred to the pressure node faster. The ultrasonic wave frequencies are controlled to separate specific particles based on the size range [61]. Furthermore, development of this technique produces highly purified EVs and can separate them from very low density lipoproteins with remarkable efficiency [65].
The other technique in this group is immuno-based microfluidic isolation. The mechanism is similar to ELISA. Compared with ELISA, smaller samples can be used in this method (microliter). The specificity of the method is related to the specificity of antibodies that are immobilized on the chip [66][67]. As mentioned before, antibodies can be loaded on the beads located on the inner surface of the microchannel [68]. To reach the mentioned specificity, Exochip has been developed recently and due to the anti-CD68 antibodies (conjugate with CD68 that is expressed on the exosomes are released via various cell types) that are fixed on the microfluidic chips, the specific EV are isolated [69]. ExoSearch is the other microfluidic chip for EV isolation that can be used for smaller samples and consumes lesser time [66]. The modified magnetic beads via specific antibodies identify CA-125, EpCAM and CD24 on the EVs of ovarian cancer [66][70].
2. Comparison of Different Methodological Isolation Procedures
The optimal isolation method is one of the greatest challenges for the clinical use of EVs. As mentioned before, various types of isolation methods have been developed. Each method had its own advantages and disadvantages. When considering different methods, “an ideal method for isolation of EVs should be relatively simple, inexpensive, should not require a complex or expensive equipment, should be relatively fast and allow for isolation of EVs from a large volume of samples” [10].
The pros and cons of each method have been described in summary in Table 1.
Table 1. Comparison of EV isolation techniques in terms of source, recovery, purity, sample volume and time.
| Method | Sources | Time | Volume | Recovery | Purity | Ref |
|---|---|---|---|---|---|---|
| Ultracentrifugation | MCF-7 cell line | 4 h | 1 mL | - | Moderate | [19] |
| Ultracentrifugation | Non-Small-Cell Lung Cancer (NSCLC) SK-MES-1 cell line | 20 h | 500 µL | 70% | <UF | [35] |
| Ultracentrifugation | Human colon carcinoma LIM1863 cells | 2h | 500 μL | 5–25% | Low | [52] |
| OptiPrep™ density gradient centrifugation | Human colon carcinoma LIM1863 cells | >21 h | 500 μL | 5–25% | >UC | [52] |
| OptiPrep™ density gradient centrifugation | human breast cancer cell line MCF-7 | 20 h | 1 mL | - | Very high | [19] |
| Density Gradient centrifugation | Tca8113 human tongue squamous cell carcinoma cell line | 20 h | >1 mL | >UC | Similar to UC | [71] |
| ExoQuick-TC™ precipitation | human breast cancer cell line MCF-7 | 13 h | 1 mL | - | Low | [19] |
| ExoChip | Blood serum | <2 h | <400 μL | Low | - | [69] |
| TEI precipitation | human breast cancer cell line MCF-7 | 13 h | 1 mL | - | Low | [19] |
| Ultrafiltration | Non-Small-Cell Lung Cancer (NSCLC) SK-MES-1 cell line | 18 h | 500 µL | 90% | >UC | [35] |
| Sequential filtration | MDA231 breast cancer cells | - | 150 mL | <UC | High | [72] |
| heparin/polymer-coated microspheres | Plasma | 1 h | 2 mL | 81% | High | [73] |
| Heat Shock Protein (HSP)-binding peptide Vn96 | HT-29 cell | 32 min | 2 mL | Poor | Poor | [74] |
| Liquid biopsy chip + HSP-binding peptide Vn96 | MCF7 | 20 min | 0.2 mL | 99% | - | [75] |
| Enzyme-linked immunosorbent assay | LNCaP cell line HCT116 cell line |
2 h | 100 μL | 75–80% | - | [9] |
| Integrated microfluidic platform | Plasma | 2 h | 30 μL | >99.9% | - | [76] |
| anti-EpCAM coated magnetic beads | Human colon carcinoma LIM1863 cells | Overnight | >1 mL | 5–25% | >UC | [52] |
| Acoustic Nanofilter | Red blood cells | <30 min | 50 μL | >80% | - | [61] |
| Microfluidic ExoSearch chip | Blood | >40 min | 20 μL | 42–97.3% | - | [66] |
| Immune-microfluidic | Cell line (ovarian cancer C30) | ~100 min | 30 μL | >99.9% | - | [67] |
| Microfluidic affinity separation chip | Serum | 20–40 min | 20–100 μL | ~60% | - | [70] |
| Micro fluidic viscoelastic flows | Serum | <5 min | <100 μL | >80% | >90% | [64] |
| Microfluidic viscoelastic flow | Blood | ∼25 min | - | > 99% | ∼98.4% | [77] |
| Double-filtration microfluidic device | Urine | <10 min | <100 μL | 74.2% | - | [78] |
| Modified acoustic | Blood | 25 min | 100 μL | 82% | 98% | [79] |
| Crossflow microfiltration | Lipo246 cell line | 30 min | - | 32–76% | Low | [80] |
| Centrifugal microfluidic | Human breast adenocarcinoma cell line, MCF-7 Lung adenocarcinoma cell line, H1975 |
<4 min | <10 μL | 90% | 85% | [81] |
The percentages of research studies published describing each isolation method are described in pie chart, Figure 2. The comparison studies have stated that UC is the primary isolation method for 41.5% of the evaluations (for source volumes from <1 to >100 mL) [82][83].
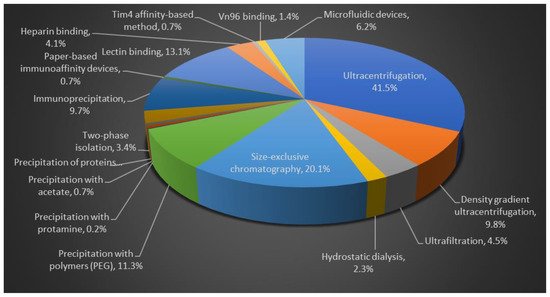
Figure 2. Comparison of different isolation methods for EVs purification. Flow chart data is based on published literature.
References
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20.
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Experientia 2019, 76, 2369–2382.
- Ullah, M.; Qian, N.P.M.; Yannarelli, G.; Akbar, A. Heat shock protein 20 promotes sirtuin 1-dependent cell proliferation in induced pluripotent stem cells. World J. Stem Cells 2021, 13, 659–669.
- Akbar, A.; Pillalamarri, N.; Jonnakuti, S.; Ullah, M. Artificial intelligence and guidance of medicine in the bubble. Cell Biosci. 2021, 11, 108.
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles 2019, 9, 1697028.
- Ullah, A.; Mabood, N.; Maqbool, M.; Khan, L.; Khan, M.; Ullah, M. SAR-CoV-2 infection, emerging new variants and the role of activation induced cytidine deaminase (AID) in lasting immunity. Saudi Pharm. J. 2021, 29, 1181–1184.
- Ullah, M.; Qian, N.P.M.; Yannarelli, G. Advances in innovative exosome-technology for real time monitoring of viable drugs in clinical translation, prognosis and treatment response. Oncotarget 2021, 12, 1029–1031.
- Pillalamarri, N.; Abdullah; Ren, G.; Khan, L.; Ullah, A.; Jonnakuti, S.; Ullah, M. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl. Oncol. 2021, 14, 101095.
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58.
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods 2018, 2, 1800021.
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65.
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.; Lazarev, V.N.; Kulemin, N.; Semina, S.E.; Generozov, E.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319.
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE 2015, 10, e0145686.
- Ullah, M.; Kodam, S.P.; Mu, Q.; Akbar, A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano 2021, 15, 3612–3620.
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; AB da Cruz e Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820.
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering 2019, 6, 8.
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10.
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol.-Ren. Physiol. 2014, 306, F1251–F1259.
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3, 24858.
- Anderson, N.G. An introduction to particle separations in zonal centrifuges. Natl. Cancer Inst. Monogr. 1966, 21, 9–39.
- Rikkert, L.G.; Engelaer, M.; Hau, C.M.; Terstappen, L.W.M.M.; Nieuwland, R.; Coumans, F.A. Rate zonal centrifugation can partially separate platelets from platelet-derived vesicles. Res. Pract. Thromb. Haemost. 2020, 4, 1053–1059.
- Kim, D.-K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2015, 113, 170–175.
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A.V. Strategies for Isolation of Exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323.
- Batrakova, E.V.; Kim, M. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405.
- Amarnath, S.; Foley, J.E.; Farthing, D.E.; Gress, R.E.; Laurence, A.; Eckhaus, M.A.; Métais, J.; Rose, J.J.; Hakim, F.T.; Felizardo, T.C.; et al. Bone Marrow-Derived Mesenchymal Stromal Cells Harness Purinergenic Signaling to Tolerize Human T h1 Cells In Vivo. Stem Cells 2014, 33, 1200–1212.
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019, 20, 240.
- Sunkara, V.; Woo, H.-K.; Cho, Y.-K. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst 2015, 141, 371–381.
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366.
- Ullah, M. Need for Specialized Therapeutic Stem Cells Banks Equipped with Tumor Regression Enzymes and Anti-Tumor Genes. J. Biomed. Allied Res. 2020, 2, 13.
- Brownlee, Z.; Lynn, K.D.; Thorpe, P.E.; Schroit, A.J. A novel “salting-out” procedure for the isolation of tumor-derived exosomes. J. Immunol. Methods 2014, 407, 120–126.
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978.
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580.
- Liga, A.; Vliegenthart, A.D.B.; Oosthuyzen, W.; Dear, J.W.; Kersaudy-Kerhoas, M. Exosome isolation: A microfluidic road-map. Lab Chip 2015, 15, 2388–2394.
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684.
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031.
- Jeppesen, D.; Hvam, M.L.; Primdahl-Bengtson, B.; Boysen, A.T.; Whitehead, B.; Dyrskjøt, L.; Ørntoft, T.F.; Howard, K.A.; Ostenfeld, M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles 2014, 3, 25011.
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.-C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723.
- Kim, K.; Park, J.; Jung, J.-H.; Lee, R.; Park, J.-H.; Yuk, J.M.; Hwang, H.; Yeon, J.H. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021, 5, 016103.
- Soda, N.; Rehm, B.H.A.; Sonar, P.; Nguyen, N.-T.; Shiddiky, M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J. Mater. Chem. B 2019, 7, 6670–6704.
- Peterson, M.F.; Otoc, N.; Sethi, J.K.; Gupta, A.; Antes, T.J. Integrated systems for exosome investigation. Methods 2015, 87, 31–45.
- Gheinani, A.H.; Vögeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945.
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, srep33641.
- Navajas, R.; Corrales, F.J.; Paradela, A. Serum Exosome Isolation by Size-Exclusion Chromatography for the Discovery and Validation of Preeclampsia-Associated Biomarkers. In Proteomics for Biomarker Discovery; Humana Press: New York, NY, USA, 2019; pp. 39–50.
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018, 16, 1.
- GH, L.; CR, R. The separation of substances on the basis of their molecular weights, using columns of starch and water. Biochem. J. 1955, 60, xxxiv.
- Ruysschaert, T.; Marque, A.; Duteyrat, J.-L.; Lesieur, S.; Winterhalter, M.; Fournier, D. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005, 5, 11.
- Vogel, R.; Coumans, F.A.W.; Maltesen, R.G.; Böing, A.N.; Bonnington, K.E.; Broekman, M.L.; Broom, M.F.; Buzás, E.I.; Christiansen, G.; Hajji, N.; et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles 2016, 5, 31242.
- Kang, D.; Oh, S.; Ahn, S.-M.; Lee, B.-H.; Moon, M.H. Proteomic Analysis of Exosomes from Human Neural Stem Cells by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography−Tandem Mass Spectrometry. J. Proteome Res. 2008, 7, 3475–3480.
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053.
- Musante, L.; Tataruch, D.; Gu, D.; Martin, A.B.; Calzaferri, G.; Aherne, S.; Holthofer, H. A Simplified Method to Recover Urinary Vesicles for Clinical Applications and Sample Banking. Sci. Rep. 2014, 4, 7532.
- Musante, L.; Tataruch, D.E.; Holthofer, H. Use and Isolation of Urinary Exosomes as Biomarkers for Diabetic Nephropathy. Front. Endocrinol. 2014, 5, 149.
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304.
- Mathivanan, S.; Lim, J.W.E.; Tauro, B.J.; Ji, H.; Moritz, R.L.; Simpson, R.J. Proteomics Analysis of A33 Immunoaffinity-purified Exosomes Released from the Human Colon Tumor Cell Line LIM1215 Reveals a Tissue-specific Protein Signature. Mol. Cell. Proteom. 2010, 9, 197–208.
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2010, 110, 13–21, Erratum in 2010, 116, 153.
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2019, 257, 3–12.
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Enzyme-Linked Immunosorbent Assay (ELISA): From A to Z; Springer: Singapore, 2018.
- Hong, C.S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation and Characterization of CD34+ Blast-Derived Exosomes in Acute Myeloid Leukemia. PLoS ONE 2014, 9, e103310.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950.
- Zhang, J.; Nguyen, L.T.H.; Hickey, R.; Walters, N.; Wang, X.; Kwak, K.J.; Lee, L.J.; Palmer, A.F.; Reátegui, E. Immunomagnetic sequential ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. Sci. Rep. 2021, 11, 8034.
- Shih, C.-L.; Chong, K.-Y.; Hsu, S.-C.; Chien, H.-J.; Ma, C.-T.; Chang, J.W.-C.; Yu, C.-J.; Chiou, C.-C. Development of a magnetic bead-based method for the collection of circulating extracellular vesicles. New Biotechnol. 2016, 33, 116–122.
- Lee, K.; Shao, H.; Weissleder, R.; Lee, H. Acoustic Purification of Extracellular Microvesicles. ACS Nano 2015, 9, 2321–2327.
- Davies, R.T.; Kim, J.; Jang, S.C.; Choi, E.-J.; Gho, Y.S.; Park, J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip 2012, 12, 5202–5210.
- Guo, S.-C.; Tao, S.-C.; Dawn, H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1508271.
- Liu, C.; Guo, J.; Tian, F.; Yang, N.; Yan, F.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano 2017, 11, 6968–6976.
- Wu, M.; Chen, C.; Wang, Z.; Bachman, H.; Ouyang, Y.; Huang, P.-H.; Sadovsky, Y.; Huang, T.J. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab Chip 2019, 19, 1174–1182.
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496.
- Jiang, C.; Fu, Y.; Liu, G.; Shu, B.; Davis, J.; Tofaris, G.K. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nanomicro Lett. 2021, 14, 3.
- Talebjedi, B.; Tasnim, N.; Hoorfar, M.; Mastromonaco, G.F.; De Almeida Monteiro Melo Ferraz, M. Exploiting Microfluidics for Extracellular Vesicle Isolation and Characterization: Potential Use for Standardized Embryo Quality Assessment. Front. Vet. Sci. 2021, 7, 1139.
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900.
- Hisey, C.L.; Dorayappan, K.D.P.; Cohn, D.E.; Selvendiran, K.; Hansford, D.J. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip 2018, 18, 3144–3153.
- Zhang, Z.; Wang, C.; Li, T.; Liu, Z.; Li, L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol. Lett. 2014, 8, 1701–1706.
- Heinemann, M.L.; Ilmer, M.; Silva, L.P.; Hawke, D.H.; Recio, A.; Vorontsova, M.A.; Alt, E.; Vykoukal, J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A 2014, 1371, 125–135.
- Mao, W.; Wen, Y.; Lei, H.; Lu, R.; Wang, S.; Wang, Y.; Chen, R.; Gu, Y.; Zhu, L.; Abhange, K.K.; et al. Isolation and Retrieval of Extracellular Vesicles for Liquid Biopsy of Malignant Ground-Glass Opacity. Anal. Chem. 2019, 91, 13729–13736.
- Knol, J.C.; de Reus, I.; Schelfhorst, T.; Beekhof, R.; de Wit, M.; Piersma, S.R.; Pham, T.V.; Smit, E.F.; Verheul, H.M.; Jiménez, C.R. Peptide-mediated ‘miniprep’ isolation of extracellular vesicles is suitable for high-throughput proteomics. EuPA Open Proteom. 2016, 11, 11–15.
- Bathini, S.; Pakkiriswami, S.; Ouellette, R.J.; Ghosh, A.; Packirisamy, M. Magnetic particle based liquid biopsy chip for isolation of extracellular vesicles and characterization by gene amplification. Biosens. Bioelectron. 2021, 194, 113585.
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 2014, 14, 3773–3780.
- Sancho-Albero, M.; Sebastián, V.; Sesé, J.; Pazo-Cid, R.; Mendoza, G.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Isolation of exosomes from whole blood by a new microfluidic device: Proof of concept application in the diagnosis and monitoring of pancreatic cancer. J. Nanobiotechnol. 2020, 18, 150.
- Liang, L.-G.; Kong, M.-Q.; Zhou, S.; Sheng, Y.-F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.-J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, srep46224.
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589.
- Casadei, L.; Choudhury, A.; Sarchet, P.; Sundaram, P.M.; Lopez, G.; Braggio, D.; Balakirsky, G.; Pollock, R.; Prakash, S. Cross-flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12062.
- Yeo, J.C.; Kenry; Zhao, Z.; Zhang, P.; Wang, Z.; Lim, C.T. Label-free extraction of extracellular vesicles using centrifugal microfluidics. Biomicrofluidics 2018, 12, 024103.
- Guerreiro, E.M.; Vestad, B.; Steffensen, L.A.; Aass, H.C.D.; Saeed, M.; Øvstebø, R.; Costea, D.-E.; Galtung, H.K.; Søland, T.M. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS ONE 2018, 13, e0204276.
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
11 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




