Soil-born plant pathogens, especially Agrobacterium, generally navigate their way to hosts through recognition of the root exudates by chemoreceptors. However, there is still a lack of appropriate identification of chemoreceptors and their ligands in Agrobacterium. Here, Atu0526, a sCache-type chemoreceptor from Agrobacterium fabrum C58, was confirmed as the receptor of a broad antibacterial agent, formic acid. The binding of formic acid to Atu0526 was screened using a thermo shift assay and verified using isothermal titration calorimetry. Inconsistent with the previously reported antimicrobial properties, formic acid was confirmed to be a chemoattractant to A. fabrum and could promote its growth. The chemotaxis of A. fabrum C58 toward formic acid was completely lost with the knock-out of atu0526, and regained with the complementation of the gene, indicating that Atu0526 is the only chemoreceptor for formic acid in A. fabrum C58.
1. Introduction
Agrobacterium fabrum is a soil-borne Gram-negative bacterium that infects a variety of dicotyledonous plants. Plants secrete organic acids and other compounds near the rhizosphere, thus forming an acidic environment
[1][2][3]. Sensing rhizospheric secretions through chemotaxis is the first step for
A. fabrum to navigate its way to the host plant
[4][5][6]. Chemotaxis permits bacteria to perceive their external surroundings (chemicals, pH, redox potential, temperature, etc.) and to swim toward a favorable environment
[7][8][9]. Chemoreceptors and the histidine kinase play essential roles in chemotactic signaling
[10][11]. Methyl-accepting chemotaxis proteins (MCPs) are the most common chemoreceptors of various ligands (attractants and repellents)
[12]. Ligand binding induces conformational changes in MCP molecules, shifting the ON-OFF equilibration of CheA auto-phosphorylation activity
[13]. CheY is thereby phosphorylated, subsequently triggering a rotation adjustment of the flagellar
[14]. The signal transduction also requires a coupling protein, CheW. CheW couples CheA to the MCP dimers to form a ternary core complex
[15][16][17]. This interaction between CheWs and MCPs is necessary for chemotactic signaling
[16].
MCPs, widely existing in bacteria, normally contain three functional elements, including a ligand-binding domain (LBD), an intervening HAMP domain and a cytoplasmic methyl-accepting (MA) signaling domain
[12]. For the MCP with periplasmic LBD, its LBD is linked to the intervening HAMP domain via transmembrane domains. The ligand-binding signal is generated by the LBD and transmitted through HAMP to the MA signaling domain
[13]. In general, the HAMP domain and the MA signaling domain are evolutionarily conserved, while LBDs display complicated diversity for the perception of various ligands and signals
[18].
Agrobacterium LBDs are distributed among six different types, including single 4HB (four-helix bundle), double 4HB, single Cache (single calcium channels and chemotaxis, sCache), double Cache (dCache), PAS (Per/Arnt/Sim) and protoglobin
[19]. Cache-type MCPs account for the largest proportion of chemoreceptors in
Agrobacterium [19]. The Cache domain was initially considered to be an extracellular PAS-like domain until more in-depth analysis and detailed classification appeared
[20]. A dCache domain is composed of two subdomains, each homologous to the PAS domain, and a long N-terminal α-helix
[21], whereas a sCache domain has only one “PAS-like” domain and a relatively short N-terminal α-helix. sCache is further divided into sCache_2, sCache_3_1, sCache_3_2 and sCache_3_3. The known ligands of all four sCache subfamilies are mainly organic acids
[20]. In
A. fabrum C58, there are seven MCP candidates belonging to the Cache superfamily, including Atu0373, Atu0526, Atu0646, Atu1912, Atu2173, Atu2223 and Atu3725
[19]. Little research has been devoted to these proteins.
Generally, the ligand-binding signal is generated by a conformational change of the LBD. The Apo- and ligand-bound 4HB-type LBD structures showed a certain degree of conformational changes
[22][23][24]. Several 4HB-type LBDs undergo an approximately 1 Å piston-like shift of the final helix (H4) when ligands bind
[25][26]. It is proposed that the ligand binding to Cache domains may also trigger conformational changes, likely by piston displacement, although the structures of Cache-type LBDs are distinct from 4HB-type LBDs
[27]. The structural analysis of Tlp3, a dCache-type MCP from
Campylobacter jejuni, suggested that the binding of an attractant to the distal subdomain locks it in a closed form, altering the proximal subdomain into an open form, which results in a 4 Å piston displacement of the C-terminal helix
[28]. For the sCache-type MCP, there is still a lack of more convincing evidence to reveal the related signal generation mechanism.
Formic acid has been used widely and long-term as a poultry feed additive to inhibit the growth of foodborne pathogens
[29]. The treatment of
E. coli with formic acid significantly reduced the synthesis rate of its DNA, RNA, protein, phospholipids and cell wall
[30]. Since formic acid is considered to be an antibacterial agent, evolutionarily, it is likely to act as a chemorepellent for bacteria. However, some studies contradict this hypothesis. For instance, formic acid was confirmed to be a chemoattractant for
C. concisus and
C. jejuni [31][32]. To date, no studies have been conducted on the chemotactic response of
A. fabrum to formic acid.
Currently, the studies of chemoreceptors for formic acid are still limited. Two adjacent genes,
cj0952c and
cj0951c, from
C. jejuni isolate B2, have been found to affect the chemotactic behavior of the strain toward formic acid
[33]. However, there is no evidence that the proteins encoded by these two genes are the chemoreceptor for formic acid. Thus far, the only confirmed chemoreceptor that binds formic acid is McpV from
Sinorhizobium meliloti [34]. However, the dissociation constant (
Kd) of McpV
PR (the LBD of McpV) binding formic acid is much higher than the
Kd of McpV
PR binding some other short-chain carboxylates. The optimal concentration of formic acid causing the chemotactic response of
S. meliloti is 100 mM, which is much higher than the concentration of formic acid occurring in natural conditions
[34]. These data indicate that formic acid interacts weakly with McpV and is an inefficient chemoattractant to
S. meliloti [34].
2. Atu0526 Is a Conserved sCache-Type MCP in Agrobacterium
Atu0526 is a protein from
A. fabrum C58 encoded by the gene annotated as
mclA. It is an HAMP domain-containing protein as described in the National Center for Biotechnology Information (NCBI) Gene database (
https://www.ncbi.nlm.nih.gov/gene accessed on 6 July 2021). The domain architecture of Atu0526 is shown in
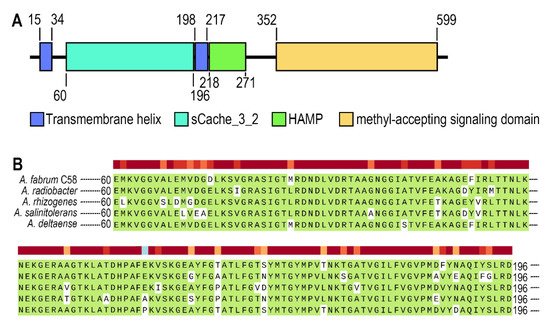
Figure 1A. Atu0526 has two transmembrane helices, a sCache_3_2 domain, an HAMP domain and a methyl-accepting signaling domain (
Figure 1A). It is not difficult to conclude that Atu0526 is likely to be a typical sCache MCP with the LBD located in the periplasm.
Figure 1. The analysis of Atu0526. (A) Domains of Atu0526 predicted by SMART server. The full-length sequence of Atu0526 was input into the SMART server for prediction, and the resulted domains were presented in the linear structure of Atu0526 as rectangles with different colors. The numbers represent the starting and ending positions of each domain. (B) sCache domain of Atu0526 was aligned with homologous proteins including Ach5_04520 from A. radiobacter, A8L48_17305 from A. rhizogenes, AGR9A_Cc210166 from A. slinitolerans and AGR7C_Cc260167 from A. deltaense. The color above the sequences represents the degree of protein conservation. The green background indicates the identical residues.
The sCache domain of Atu0526 was also aligned with those of five homologous MCPs from A. radiobacter, A. rhizogenes, A. salinitolerans and A. deltaense. The alignment showed a high similarity among these sequences, suggesting that the proteins are highly conserved in Agrobacterium species (Figure 1B). The high degree of conservation may indicate the basic role of the protein in Agrobacterium.
3. Atu0526 Interacts with both CheW1 and CheW2
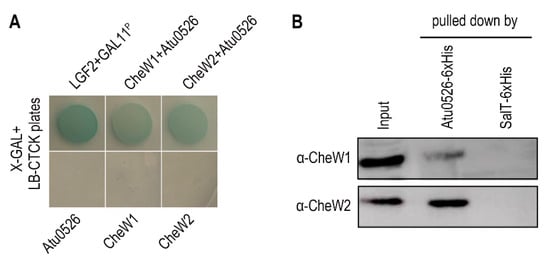
The interaction with the CheW proteins, as a basic characteristic of the functional MCP, was also tested through bacterial two-hybrid and pull-down experiments. A. fabrum C58 has two CheW proteins, CheW1 and CheW2. The bacterial two-hybrid and pull-down experiments confirmed the interaction between Atu0526 and CheW1, and Atu0526 and CheW2 (Figure 2A,B).
Figure 2. The interaction between Atu0526 and CheWs. (
A) Bacterial two-hybrid for testing the interactions between Atu0526 and CheWs. The bacterial cells harboring positive control proteins or different combinations of Atu0526 and CheWs were spotted on LB-CTCK plates with X-GAL. Bacterial cells can grow on the plate and appear blue, indicating an interaction between proteins. (
B) Pull-down assay for testing the interactions between Atu0526 and CheWs. The 6×His-tagged Atu0526 and SalT were incubated with the crude extraction of
A. fabrum C58 and then pulled down by Ni-IDA Resins. The eluted solutions were subsequently immunoblotted with the antibodies against two CheWs
[35] Original blot images in
Supplementary Figures S1 and S2.
4. LBD of Atu0526 Binds Formic Acid
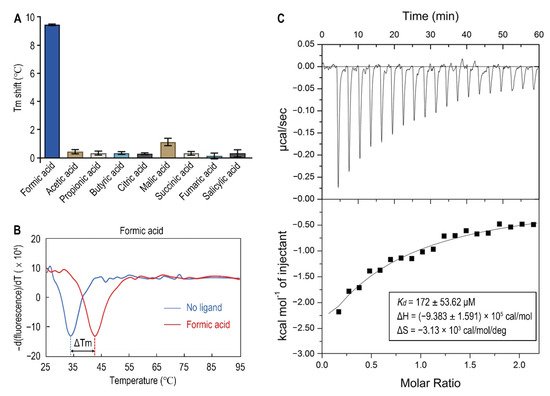
Since organic acids are the major known ligands for sCache receptors
[20], nine organic acids were tested as candidates of Atu0526 ligands using a thermo shift assay. As the results showed, the melting temperature (T
m) showed different shift amplitudes in the presence of different ligands (
Figure 3A). Generally, a T
m shift of more than 2 °C induced by a ligand is considered significant
[36]. The T
m of Atu0526
LBD was 33.40 ± 0.62 °C while the T
m of Atu0526
LBD with formic acid added reached 42.42 ± 0.41 °C (
Figure 3B). This significant T
m shift means that the addition of formic acid greatly enhanced the stability of Atu0526
LBD, indicating a strong binding of formic acid to the protein. Meanwhile, other organic acids showed no significant T
m shift, suggesting a specificity of Atu0526 in binding to formic acid.
Figure 3. The binding of formic acid to Atu0526LBD. (A) Thermo shift assay of different organic acids. The column values represent the Tm of Atu0526LBD with the corresponding ligands minus the Tm without ligand. Column values are the means of three biological replicates with the SD. (B) The binding analysis of formic acid to Atu0526LBD using a thermo shift assay. The data are presented in the form of changes in the derivative of fluorescence intensity with temperature. The temperature corresponding to the red and blue dashed lines are the Tm values of Atu0526 with and without formic acid, respectively. (C) Isothermal titration calorimetry of Atu0526LBD with formic acid. The upper panel depicts the raw titration data. The lower panel is the isotherm derived by integrating peaks from the raw data and the calculated values of Kd, ∆H and ∆S. Data points were fitted with the “one binding site model” of ORIGIN.
In order to obtain direct evidence of the binding between formic acid and Atu0526LBD, isothermal titration calorimetry (ITC) was applied. The results confirmed the binding, resulting in an exothermic reaction with a Kd of 172 ± 53.62 μM (Figure 3C). The enthalpy change (ΔH) for the reaction was (−9.383 ± 1.591) ×105 cal/mol, and the entropy change (ΔS) for the reaction was −3.13 × 103 cal/mol/deg (Figure 3C). According to Gibbs free energy calculations (ΔG = ΔH − TΔS), the reaction was spontaneous. Moreover, the values of ΔH and ΔS were all negative, suggesting that hydrogen bond could be the main driving force in this binding reaction.
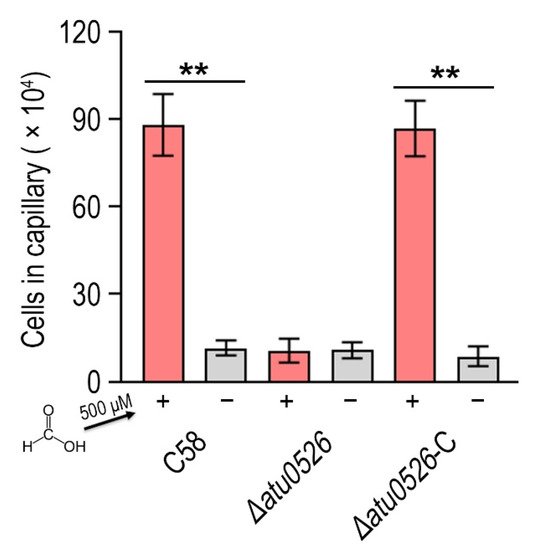
5. Formic Acid Is a Chemoattractant of A. fabrum C58
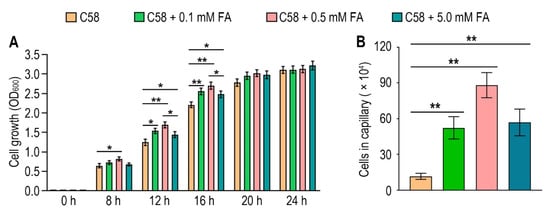
To identify the possible effect of formic acid on A. fabrum, we first tested the growth of A. fabrum C58 in the presence of different concentrations of formic acid. Contrary to the situation in some other bacteria, the growth of A. fabrum C58 was significantly (p < 0.05) promoted in the presence of formic acid (Figure 4A), implying that formic acid is beneficial to A. fabrum growth and may be a potential chemoattractant of A. fabrum. To verify whether A. fabrum could be attracted by formic acid, we tested the chemotactic response of A. fabrum to different concentrations of formic acid by using a capillary assay. Our results confirmed that A. fabrum C58 was significantly (p < 0.01) chemoattracted by formic acid. The bacterial population chemoattracted in the capillary tube containing 0.5 mM formic acid was 7.6 times that in the capillary tube without formic acid (Figure 4B).
Figure 4. Formic acid can promote the growth of A. fabrum C58 and act as its chemoattractant. (A) Formic acid promoted the growth of A. fabrum C58. To the initial bacterial culture with OD600 = 0.03, 0, 0.1 mM, 0.5 mM or 5 mM formic acid were added. OD600 of each culture was measured after incubation for 8, 12, 16, 20 and 24 h. (B) Capillary assay of A. fabrum C58 for formic acid. In the capillary assay, 0, 0.1 mM, 0.5 mM and 5 mM formic acid were applied. Data are the means of three biological replicates with the SD in (A) and ten biological replicates with the SD in (B). Significant difference (p < 0.05) is indicated by * and significant difference (p < 0.01) is indicated by ** in (A,B).
6. Atu0526 Is the Only Chemotactic Receptor of A. fabrum C58 to Recognize Formic Acid
Now it has been confirmed that A. fabrum C58 is chemoattracted by formic acid and Atu0526LBD can bind formic acid, we needed to confirm whether Atu0526 was the chemoreceptor regulating the chemotaxis of A. fabrum toward formic acid. To demonstrate the role of Atu0526 in regulating A. fabrum chemotaxis, we constructed two A. fabrum derivatives, atu0526-deficient mutant (∆atu0526) and the complementary strain of ∆atu0526 (Δatu0526-C). The Capillary assay showed that the chemotaxis of A. fabrum C58 toward formic acid was completely abolished after atu0526 was knocked out (Figure 5), suggesting that Atu0526 is the only chemoreceptor for formic acid in A. fabrum C58. Additionally, the complementary strain Δatu0526-C showed the same chemotactic phenotype for formic acid as the wild type strain (Figure 5). Combined with the previous results of the in vitro binding experiments, it can be concluded that Atu05256 is a specific chemoreceptor for formic acid.
Figure 5. The effect of atu0526 on the chemotaxis of A. fabrum toward formic acid. A. fabrum C58, ∆atu0526, ∆atu0526-C were tested in a capillary assay with 500 µM formic acid. + Represents 500 µM formic acid was applied, while—represents the case without formic acid. Data are the means of ten biological replicates with the SD. Significant differences (p < 0.01) are indicated by **.
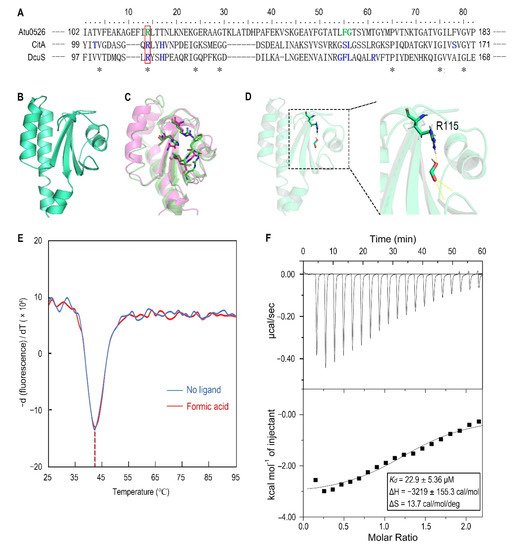
7. Replacement of R115 by Alanine Significantly Increases the Stability of Atu0526LBD and the Affinity to Formic Acid
To identify the amino acid residues that are directly involved in the binding of Atu0526
LBD to formic acid, the Atu0526 sCache_3_2 domain was aligned with the corresponding domains of the two typical sCache-type receptors, CitA from
Klebsiella pneumoniae and DcuS from
E. coli (
Figure 6A). Both CitA and DcuS are transmembrane sensors containing a sCache_3_2 domain like Atu0526. The structures of CitA and DcuS LBD domains with ligands have all been resolved
[37][38]. The important residues of CitA and DcuS at the interface between proteins and ligands were compared with those of Atu0526. It was noted that the residues of CitA and DcuS that bind ligands are relatively conserved in certain regions (
Figure 6A,C). Among these residues, only the arginine at position 115 of Atu0526 was identical to the corresponding residues of CitA and DcuS, suggesting that this residue may play an essential role in the binding. To further scrutinize the residues related to ligand binding, the 3D structure of Atu0526
LBD was predicted by the PHYRE2 server, and this protein structure was then interrogated for a possible interaction with formic acid using molecular dynamics modeling. As shown in the docking model, formic acid might form hydrogen bonds with R115, F156 (phenylalanine at position 156) and G157 (glycine at position 157) (
Figure 6D). These residues are in the same ligands binding regions as CitA and DcuS (
Figure 6A), and it once again hinted that R115 may be important for ligand binding. However, it should be pointed out that there may be other binding-related residues that could not be identified in the predicted model due to the lack of resolved crystal structure.
Figure 6. The analysis of R115 on the binding of formic acid to Atu0526LBD. (A) Sequence alignment of sCache_3_2 domains of Atu0526, CitA and DcuS. * refers to identical residues. The residues shown in blue are residues of CitA and DcuS ligand binding. The residues shown in green were predicted to bind formic acid. The red rectangle indicates R115. (B) The predicted 3D structure of sCache_3_2 domain of Atu0526. (C) The 3D structures of sCache domains from CitA (magenta, 1P0Z) and DcuS (green, 3BY8). The important residues that ligands bind to are shown in stick form. (D) The docking of formic acid with Atu0526LBD. The right part is an enlarged presentation of the content in the dashed box. The hydrogen bonds between the ligand and the protein are represented by yellow dashed lines. The R115 is in the stick diagram. (E) The binding analysis of formic acid to Atu0526LBDR115A using a thermo shift assay. The data are presented in the form of changes in the derivative of fluorescence intensity with temperature. The temperatures corresponding to the red and blue dashed lines are the Tm values of Atu0526LBDR115A with and without formic acid, respectively. (F) Isothermal titration calorimetry of Atu0526LBDR115A with formic acid. The upper panel depicts the raw titration data. The lower panel is the isotherm derived by integrating peaks from the raw data and the calculated values of Kd, ∆H and ∆S. Data points were fitted with the “one binding site model” of ORIGIN.
For R115, the atom predicted to form a hydrogen bond with formic acid is provided by the side group of R115 (Figure 6D). Therefore, we replaced this arginine with alanine to remove the potential force. This single-residue mutated Atu0526LBD variant was designated as Atu0526LBDR115A. For F156 and G157, the atoms predicted to form hydrogen bonds with formic acid are provided by the peptide bonds of F156 and G157, not by the side groups. A single-residue mutation to F156 or G157 has no potential significance for the binding analysis. Thus, our study focused on R115. The purified Atu0526LBDR115A was then tested in thermo shift assay. The Tm of Atu0526LBDR115A was 42.19 ± 0.39 °C which was much higher than that of Atu0526LBD (Figure 3B and Figure 6E), indicating that the single-residue mutation increased the protein stability. However, the Tm of Atu0526LBDR115A only rose by 0.24 °C to 42.43 ± 0.32 °C after adding formic acid (Figure 6E). That was to say, the addition of formic acid did not change the stability of Atu0526LBDR115A significantly.
Since adding formic acid did not change the stability of Atu0526LBDR115A, did this mean that formic acid would not bind to Atu0526LBDR115A? With this question in mind, we conducted an isothermal titration calorimetry to test the binding of formic acid to Atu0526LBDR115A. As the results showed, formic acid could bind to Atu0526LBDR115A with a Kd of 22.9 ± 5.36 μM (Figure 6F). The Kd was significantly (p < 0.01) lower than that of formic acid binding to Atu0526LBD, which means Atu0526LBDR115A has a stronger affinity with formic acid than Atu0526LBD. Although we did not initially expect that Atu0526LBDR115A would bind to formic acid, the binding of formic acid to Atu0526LBD had indeed been changed due to the mutation.
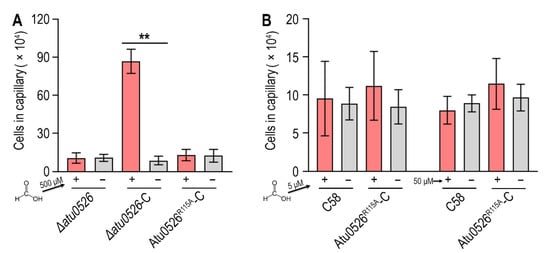
8. Replacement of R115 by Alanine Completely Destroys the Function of Atu0526 in Regulating Chemotaxis, but Not the Cellular Localization
In order to study whether the binding of formic acid to Atu0526LBDR115A can correctly trigger a chemotactic response, we introduced the R115A mutation into ∆atu0526-C and conducted a capillary assay to assess the chemotactic motility toward formic acid. The results showed that the strain with R115Atu0526 replaced by alanine (Atu0526R115A-C) was no longer chemotactic toward 500 μM formic acid (Figure 7A). The phenotype of Atu0526R115A-C was consistent with Δatu0526 (Figure 7A). That means that although formic acid can bind to Atu0526LBDR115A, this binding does not result an effective chemotactic response at a formic acid concentration of 500 μM.
Figure 7. The effect of R115 on the chemotaxis of A. fabrum C58 toward formic acid. (A,B) Capillary assay of strains for formic acid. In (A), ∆atu0526, ∆atu0526-C and Atu0526R115A-C were tested in capillary assay with 500 µM formic acid. In (B), A. fabrum C58 and Atu0526R115A-C were tested with 5 µM and 50 µM formic acid. + Represents 5, 50, or 500 µM formic acid was applied, while − represents the case without formic acid. Data in (A,B) are the means of ten biological replicates with the SD. Significant differences (p < 0.01) are indicated by **.
Since Atu0526LBDR115A has a stronger affinity with formic acid, we reduced the formic acid concentration by one and two orders of magnitude in the capillary assay to test if Atu0526R115A could sense a lower concentration of formic acid. The results showed that neither 5 nor 50 μM formic acid could trigger the chemotactic response of Atu0526R115A-C (Figure 7B), suggesting that the R115 mutation does not make a more sensitive chemoreceptor, but just a non-functional chemoreceptor for formic acid.
In order to check the possibility that the R115 mutation affects the protein localization, thereby affecting the chemotaxis toward formic acid, we fused green fluorescent protein (GFP) with Atu0526 and Atu0526
R115A, and expressed the GFP fused proteins in
∆atu0526. As shown in the pictures, Atu0526-GFP and Atu0526
R115A-GFP showed similar localization (
Supplementary Materials Figure S3), indicating that R115 does not affect the localization of Atu0526.
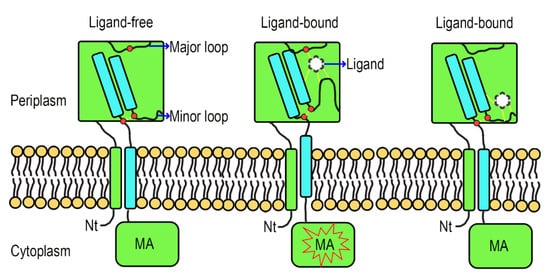
9. A Hypothetical Signal Transduction Model of Atu0526
Formic acid can bind to Atu0526
R115A but cannot trigger a chemotactic response of Atu0526
R115A-C, and the localization of Atu0526 is not altered by the residue substitution. Therefore, we suspect that R115 plays a role in the signal transduction of Atu0526. Although the studies of the signal transduction mechanism of sCache-type MCPs are limited, the studies of the non-MCP sCache-type receptors allow to formulate a working hypothesis. The resolved structures of citrate-free and citrate-bound CitA indicate a presence of signal generating mechanism
[39]. In CitA, a minor loop has a maximum displacement of 13.54 Å due to the binding of citrate, and the displacement drives the adjacent β-sheet to curl toward the center, thus generating the signal (
Supplementary Materials Figure S4)
[39]. Several other sCache-type receptors and the molecular dynamic modeling predicted Atu0526 also conform to this mechanism
[38][40]. In our molecular dynamic modeling, formic acid binds to the R115, F156 and G157 of Atu0526 (
Figure 6D). R115 is in the major loop (the loop connecting the first and second β-sheets of Atu0526
LBD), while F156 and G157 are in the minor loop (the loop connecting the second and third β-sheets of Atu0526
LBD). The minor loop is generally flexible in the sCache_3_2 domain
[37][38][39]. Therefore, we conjecture that formic acid binds to R115, and then F156 and G157 binds to the formic acid, pulling the minor loop toward the major loop. Such a speculative way of ligand-binding would cause the conformational change of Atu0526
LBD to generate the signal.
To make this clear, a hypothetical model showing the signal transduction of Atu0526 was prepared. In this model, when there is no ligand binding to the Atu0526, the minor loop is in a free stretch state, and the C-terminus of the LBD will not form enough movement to induce the MA signaling (Figure 8). When a ligand binds to the LBD correctly, the ligand-binding residues (such as R115) in the major loop act as anchorages that will pull the minor loop closer through the ligand and drive the Atu0526LBD into a closed conformation. This conformational change will cause an upward displacement of the LBD C-terminus, thereby activating the cytoplasmic MA signaling (Figure 8). If there are not enough anchorage residues in the chemoreceptor (Atu0526R115A), the ligand may still bind to the LBD. However, such a binding is not able to form a closed conformation and induce MA signaling (Figure 8). This model may explain why Atu0526R115A can bind formic acid but cannot cause a chemotactic response to formic acid.
Figure 8. A hypothetical signal transduction model of Atu0526. The formic acid anchors to the anchorage residues (such as R115) and pulls the minor loop upward to realize chemotactic signal transduction. When the anchorage residues are not enough, the ligand may be able to bind to Atu0526, but it is not sufficient to induce a conformational change for chemotactic signal transduction. The red dot above refers to anchorage residue(s), and the two red dots below refer to the C-terminal residues of the fourth β-sheet and the nearby minor loop residues. The force between ligand and Atu0526 or between two residues is indicated by yellow dashed lines.