Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rokeya Sultana | + 1982 word(s) | 1982 | 2022-01-04 09:48:24 | | | |
| 2 | Jason Zhu | Meta information modification | 1982 | 2022-01-07 03:02:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sultana, R. Thymoquinone in Chemotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/17815 (accessed on 07 February 2026).
Sultana R. Thymoquinone in Chemotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/17815. Accessed February 07, 2026.
Sultana, Rokeya. "Thymoquinone in Chemotherapy" Encyclopedia, https://encyclopedia.pub/entry/17815 (accessed February 07, 2026).
Sultana, R. (2022, January 06). Thymoquinone in Chemotherapy. In Encyclopedia. https://encyclopedia.pub/entry/17815
Sultana, Rokeya. "Thymoquinone in Chemotherapy." Encyclopedia. Web. 06 January, 2022.
Copy Citation
Thymoquinone (2-Isopropl-5-methyl benzo-1,4-quinone) is an active component of the volatile oil of Nigella sativa (black seeds). Other than the Ranunculaceae family (N. sativa), this compound has been detected in other families such as Lamiaceae (Modernadidyma, M. menthifolia, etc.), Asteraceae (Eupatorium cannabinum), and Cupressaceae (Juniperus communis).
Thymoquinone
chemotherapy-induced toxicity
antioxidant
1. Thymoquinone (TQ)
Thymoquinone has a broad range of pharmacological actions involving anti-oxidative, anti-inflammatory, immunomodulatory, anti-cancer, anti-microbial, hepatoprotective, hypoglycemic and antidiabetic, gastroprotective, neuroprotective, cardioprotective, nephroprotective, hypolipidemic, and anti-histaminic effects. It also showed a protective effect on reproductive system, respiratory system, and bone related disorders [1]. TQ is generally safe to use but toxicity has been observed at higher doses (LD50, 2.5 g/kg). In a study conducted by Abukhader, Wistar Albino rats administered with 30 mg/kg and 40 mg/kg of TQ showed signs of toxicity such as lethargy, abdominal swelling, piloerection, irritability, and weight loss. It was found that 22.5 mg/kg and 15 mg/kg are the maximum tolerated doses (MTD) through the intraperitoneal route (I.P) in male rats and female rats, respectively. When administered orally at doses of 500 mg/kg and 300 mg/kg, toxicity signs like dyspnea, abdominal distension, weight loss, diarrhea, and hypoactivity were observed. The MTD through oral route (P.O) for both male as well as female rats were reported as 250 mg/kg. MTD is the highest safe dose that can be administered without showing signs of toxicity [2].
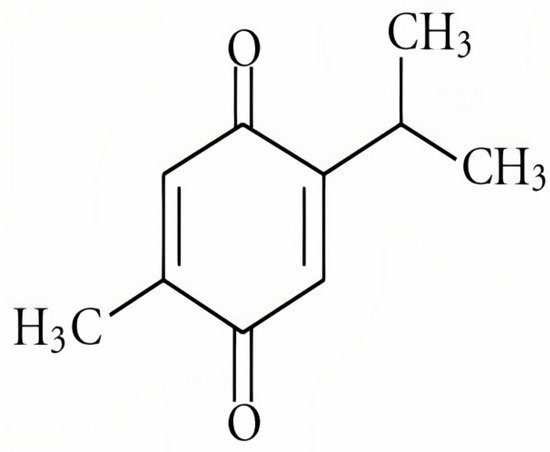
Figure 1. The chemical structure of thymoquinone [3].
1.1. Role of Thymoquinone in Cancer
There are many preclinical studies (both in vivo and in vitro) which demonstrate the effect of TQ, given alone or in combination with other chemotherapeutic agents. It is known to target various processes of the cancer model such as cell cycle progression, proliferation, apoptosis, angiogenesis, migration, invasion, and metastasis of tumors. It also prevents oxidative damage and inhibits inflammatory responses [1]. TQ showed therapeutic efficacy against a wide range of cancer including ovarian cancer [6][7], breast cancer [8][9], pancreatic cancer [10], lung cancer [11][12], fibrosarcoma [13], neuroblastoma [14], osteosarcoma [15], myeloma [16], oral cancer [17], colon cancer [18][19], prostate cancer [20], squamous cell carcinoma [21], gastric cancer [22], leukemia [23], cervical cancer [24], liver cancer [25][26], and skin cancer [27]. In addition to its chemotherapeutic effects, it also diminishes the toxic side effects caused by chemotherapeutic agents.
1.2. Effect of TQ against ChemotherapyInduced Organ Toxicity
Toxicity is a major concern for chemotherapeutic drugs. Some of the most widely used chemotherapeutic drugs possess organ toxicity which leads to further economic burden and decreased quality of life. Isolated phytoconstituents are equipotent to synthetic analogues and have lesser side effects.
1.2.1. Protective Effect against Cardiotoxicity
Many studies have demonstrated that TQ has a cardioprotective effect against chemotherapeutic drug-induced cardiotoxicity. Doxorubicin is an antitumor antibiotic used in the treatment of various types of cancer but its use is restricted due to the dose limiting cardiotoxicity. Alam et al. [28] conducted a study which demonstrated that serum enzyme markers of cardiotoxicity such as AST, ALT, CK-MB, and LDH in doxorubicin treated groups were elevated. MDA, a final product of lipid peroxidation, which act as an indicator for oxidative stress was increased and antioxidant enzymes such as CAT, SOD, GPx, GR, GST and GSH were decreased in doxorubicin treated groups. Oxidative stress occurs due to increase in reactive oxygen species generation and/or weakened antioxidant defense mechanism. Upon TQ treatment in mice (10 mg/kg, 20 mg/kg p.o), elevated levels were restored to normal. They also suggested that restoration may be due to the high antioxidant property of thymoquinone in the myocardium, thus minimizing the damage to the cardiac muscle fibers caused by doxorubicin. Co-treatment with TQ also restored the elevated levels of inflammatory cytokine (IL2) in cardiac tissue. These results were in accordance with other studies on doxorubicin-induced cardiotoxicity [29][30][31]. Karabulut et al. [31] performed a histopathological examination which revealed myocardial fibril disorganization in DOX-treated groups which was almost normally organized after treatment with TQ (10 mg/kgi.p). ANP and NT-proBNP values were determined, revealing that a doxorubicin dose of 15 mg/kg, i.p resulted in cardiac dysfunction leading to heart failure. These values were decreased by administration of thymoquinone before and after DOX-treatment. TQ supplementation also prevented cyclophosphamide-induced cardiotoxicity by restoring the abnormal levels of serum cardiac markers, lipid peroxidation, antioxidant enzymes and inflammatory mediators [32]. Cisplatin treatment caused myocardial tissue necrosis and apoptosis as evidenced by histopathological examination, which was diminished after TQ administration [33].
1.2.2. Protective Effect against Hepatotoxicity
Tamoxifen is commonly recommended for treatment of patients with breast cancer [34]. It has the unusual side effect of producing hepatotoxicity, making long-term use challenging. Treatment with tamoxifen to rats resulted in significant increase in ALT, ALP, AST, LDH, γGT and total bilirubin. Histopathological changes included swelling of interstitial tissues and inflammation of Von Kuppfer cells with atrophy. There was a significant increase in lipid peroxidation level, inflammatory marker (TNF-α) and a significant decrease in antioxidant levels. All these changes ameliorated after administration of TQ [35]. It also showed hepatoprotective activity against cisplatin, cyclophosphamide and methotrexate toxicity with similar improvements in hepatic serum markers, lipid peroxidation, inflammatory markers, and antioxidant levels [36][37][38]. As a defence mechanism, inducible nitric oxide synthase (iNOs) is known to produce significant levels of nitric oxide; however, the excess production may cause damage to the liver. El-Sheikh et al. [38] performed immunohistochemical staining of the liver in methotrexate-treated rats and it was observed that iNOs expression upregulated, which confirmed nitrosative stress. Nitric oxide levels were also increased in cisplatin-induced hepatotoxicity [36]. These effects were reversed after treatment with TQ, suggesting that it has an antinitrosative effect. All these data show that TQ has hepatoprotective activity and can be used as an adjuvant in treating hepatotoxicity caused by chemotherapeutic drugs.
1.2.3. Protective Effect against Nephrotoxicity
Literature survey revealed the protective effect of thymoquinone against nephrotoxicity in rats when given in combination with doxorubicin, cisplatin and ifosfamide. Badary et al. [37] demonstrated that doxorubicin treatment produced renal changes as manifested by hypoproteinemia, proteinuria, albuminuria, hyperlipidemia, hypoalbuminemia. They also evaluated oxidative stress as examined by lipid peroxide generation, non-protein sulfhydryl (NPSH) concentration and catalase (CAT) activity in renal tissue. These changes were improved after TQ administration to nephritic rats. A study was conducted on both rats as well as mice by Badary et al. [38] revealing that the rats were more sensitive to cisplatin-induced nephrotoxicity than mice. It was found that serum urea and serum creatinine levels were elevated with decreased creatinine clearance, increased urinary volume and renal tubular damage was also observed in the cisplatin treated group. Similar changes were also observed in ifosfamide-induced nephrotoxicity [39]. Upon TQ administration, the abnormal levels were restored to normal. TQ showed protection against doxorubicin-induced nephrotoxicity by improving lipid peroxidation, antioxidant levels, nitrosative stress markers as well as inflammatory markers [40].
1.2.4. Protective Effect against Intestinal Toxicity
Methotrexate is known to cause serious intestinal toxicity. A study has demonstrated the mechanism by which TQ shows protection against methotrexate-induced intestinal toxicity. It acts by decreasing oxidative and nitrosative stress markers in intestinal mucosa, decreasing expression of inflammatory markers (TNF-α, NF-κB and COX-2) and by inhibiting apoptosis [41]. Another study by Shahid et al. [42] estimated the effect of long-term cisplatin use in rat intestines and the role of TQ in preventing toxicity. It was observed that repeated doses of cisplatin increased MDA levels, decreased GSH and TSH levels, as well as other antioxidant enzymes such as SOD, GSH-Px, CAT, GST, GR and TR in intestinal mucosa. Glutathione peroxidase, glutathione reductase, and glutathione-s-transferase are the main GSH-dependent antioxidant enzymes. Histopathology of brush border mucosa (BBM) revealed morphological changes such as congestion, swelling of villi, alteration in the contour and increased lymphocytic infiltration in the lamina propria associated with reduction in the crypt/villus ratio. It was further noticed that there was a significant reduction in enzyme markers of brush border mucosa which are sucrase, ALP, GGTase, and LAP. They also observed alterations in enzymes involved in carbohydrate metabolism. These abnormalities in both methotrexate and cisplatin were restored to normal upon TQ administration.
1.2.5. Protective Effect against Urotoxicity
Cyclophosphamide-induced hemorrhagic cystitis is the dose limiting toxicity causing damage to urothelium of bladder mucosa. However, administration of TQ has shown protective activity against it by increasing antioxidant enzymes, decreasing lipid peroxidation, reducing levels of inflammatory markers, and maintaining the structure and morphology of the urinary bladder by increasing Nrf2 expression. Oxidative stress causes the level of Nrf2 to decrease [43].
1.2.6. Protective Effect against Ototoxicity
Sagit et al. [42] demonstrated that cisplatin-induced ototoxicity in rats is caused due to an increase in ABR thresholds and a decrease in DPOAE responses. The intensity of sound at which a brain response initially appears is determined by ABR analysis [44]. DPOAEs are emission of sounds in response to two tons of different frequencies played at the same time [45]. Accumulation of cisplatin in cochlear tissues generates excessive free radicals and decreases antioxidant enzymes which results in cochlear injury or cell death. TQ administration preserved the changes produced by cisplatin treatment [46].
1.2.7. Protective Effect against Testicular Injury
Gokce et al. [47] identified rise in TAC level and myeloperoxidase activity in methotrexate treated groups. An increase in TAC is a result of compensation to oxidative stress generated while increased myeloperoxidase activity indicates neutrophil accumulation leading to oxidative testicular damage. The seminiferous epithelium was severely disrupted, reducing the diameter of the seminiferous tubules and affecting the spermatogenetic cell lines. It also caused interstitial space dilation and edema in mice with cell size reduction and cytoplasmic swelling. TQ exerted its protective effect by restoring the abnormalities caused produced by methotrexate treatment.
1.2.8. Protective Effect against Pulmonary Toxicity
A study was conducted by Suddek et al. [48] to demonstrate acute pulmonary toxicity induced by cyclophosphamide. They observed increase in MDA levels, SOD (as a compensatory mechanism), total protein, serum LDH, TNF-α and a decrease in GSH. Histopathological examination of the lung revealed congestion, damage, swelling of interalveolar septum, neutrophilic, and macrophages infiltration. TQ supplementation resulted in reversal of the changes produced by Cyclophosphamide.
2. Role of Thymoquinone against Chemotherapy Induced Oxidative Stress
Reactive oxygen species (ROS) are produced as a result of various cellular processes involving endogenous and exogenous pathways, constituting oxidative stress [49]. Endogenous pathways include oxidative phosphorylation, bacterial invasion, and inflammation; whereas exogenous pathway includes xenobiotics, radiation, and pollution. ROS are implicated in the anticancer mechanism of chemotherapy which further causes adverse effects. In other words, it has been established that chemotherapeutic agents have the potential to induce oxidative stress during the treatment [50]. Conklin [51] reported that among anticancer drugs, oxidative stress was observed to be the highest in theanthracycline class of drugs followed by platinum compounds, alkylating agents, epipodophyllotoxins and camptothecins but was lower in taxanes, antimetabolites, and vinca alkaloids. Hence the generation of ROS following the administration of chemotherapeutic agents decreases their efficacy. Inorder to prevent the side effects and to enhance responsiveness to therapy, antioxidants are recommended. SOD and CAT are antioxidant enzymes which are primarily responsible for destroying reactive oxygen metabolites. Glutathione enzymes (GSH, GPx, GR, GST) have an important role in providing secondary defense against oxidative damage caused by the generation of ROS [52]. Previous studies described how the supplementation of thymoquinone to experimental animals treated with chemotherapeutic agents increased antioxidant enzyme activity and thus showed some protection against organ damage.
3. New Trends and Directions of Research Related to TQ
Due to poor pharmacokinetic characteristics of TQ, its use in humans is limited as it is rapidly eliminated and slowly absorbed. To enhance bioavailability, several researchers developed nanoformulations of TQ which showed marked improvement in pharmacokinetic properties. In 2017, the United States government registered a clinical trial which evaluated the chemopreventive effect of TQ on oral potentially malignant lesions. Currently there are three completed trials and two ongoing trials registered for thymoquinone [53].However, there are no registered clinical trials on role of thymoquinone in reducing the toxicity of anticancer drugs. There are sufficient in vitro and in vivo studies describing the potential of TQ in reducing chemotherapeutic drug-induced toxicity, hence undertaking future clinical investigation in this particular area would be interesting in order to improve the efficacy of chemotherapy by diminishing the adverse effects produced during treatment.
References
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158.
- Abukhader, M.M. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J. Pharm. Sci. 2012, 74, 195–200.
- Aras, S.; Gerin, F.; Aydin, B.; Ustunsoy, S.; Sener, U.; Turan, B.C.; Armutcu, F. Effects of sodium arsenite on the some laboratory signs and therapeutic role of thymoquinone in the rats. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 658–663.
- Available online: https://commons.wikimedia.org/wiki/File:Nigella_sativa_002.JPG (accessed on 20 November 2021).
- Melissa, P. “black cumin”. Encyclopedia Britannica. 2018. Available online: https://www.britannica.com/plant/black-cumin (accessed on 18 November 2021).
- Taha, M.M.; Sheikh, B.Y.; Salim, L.Z.; Mohan, S.; Khan, A.; Kamalidehghan, B.; Ahmadipour, F.; Abdelwahab, S.I. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell Mol. Biol. 2016, 62, 97–101.
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tu-mor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46.
- Yıldırım, I.H.; Azzawri, A.A.; Duran, T. Thymoquinone induces apoptosis via targeting the Bax/BAD and Bcl-2 pathway in breast cancer cells. Dicle Tıp Derg. 2019, 46, 411–417.
- Rajput, S.; Kumar, B.N.; Dey, K.K.; Pal, I.; Parekh, A.; Mandal, M. Molecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013, 93, 783–790.
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 341.
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. Journal of cellular physiology. J. Cell Physiol. 2019, 234, 10421–10431.
- Hussein, S.A.; Abdel-Aal, S.A.; Amin, A.; Khalaf, H.A. Caspase-3, Bcl-2, p53, CYP1A1 and COX-2 as a potential target in chemoprevention of Benzo (a) pyrene-induced lung carcinogenesis in mice: Role of thymoquinone. Nat. Sci. 2016, 4, 430–441.
- Badary, O.A.; Gamal El-Din, A.M. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcomatumorigenesis. Cancer Detect Prev. 2001, 25, 362–368.
- Arumugam, P.; Subramanian, R.; Priyadharsini, J.V.; Gopalswamy, J. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin. J. Nat. Med. 2016, 14, 904–912.
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.; Yang, S.; Ying, X.; Shen, W. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578.
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2013, 5, 634–648.
- Abdelfadil, E.; Cheng, Y.H.; Bau, D.T.; Ting, W.J.; Chen, L.M.; Hsu, H.H.; Lin, Y.M.; Chen, R.J.; Tsai, F.J.; Tsai, C.H.; et al. Thymoquinone induces apoptosis in oral cancer cells through p38β inhibition. Am. J. Chin. Med. 2013, 41, 683–696.
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828.
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.Y.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone Induces Caspase-Independent, Autophagic Cell Death in CPT-11- Resistant LoVo Colon Cancer via Mitochondrial Dysfunction and Activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546.
- Singh, S.K.; Apata, T.; Gordetsky, J.B.; Singh, R. Docetaxel Combined with Thymoquinone Induces Apoptosis in Prostate Cancer Cells via Inhibition of the PI3K/AKT Signaling Pathway. Cancers 2019, 11, 1390.
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; Choudhury, S.M.; Kundu, S.C.; Mandal, M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgeninin squamous cell carcinoma. PLoS ONE 2012, 7, e46641.
- Feng, L.M.; Wang, X.F.; Huang, Q.X. Thymoquinone induces cytotoxicity and reprogramming of EMT ingastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017, 42, 547–554.
- Musalli, M.G.; Hassan, M.A.; Sheikh, R.A.; Kalantan, A.A.; Halwani, M.A.; Zeyadi, M.; Hosawi, S.; Alhosin, M. Thymoquinone induces cell proliferation inhibition and apoptosis in acute myeloid leukemia cells: Role of apoptosis-related WT1 and BCL2 genes. Eur. J. Cell Sci. 2019, 1, 2–9.
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37–42.
- Meral, I.; Pala, M.; Akbas, F.; Ustunova, S.; Yildiz, C.; Demirel, M. Effects of thymoquinone on liver miRNAs and oxidative stress in Ehrlich acid mouse solid tumor model. Biotech. Histochem. 2018, 93, 301–308.
- Bashir, A.O.; El-Mesery, M.E.; Anwar, R.; Eissa, L.A. Thymoquinone potentiates miR-16 and miR-375 expressions in hepato-cellular carcinoma. Life Sci. 2020, 254, 117794.
- Park, J.E.; Kim, D.-H.; Ha, E.; Choi, S.M.; Choi, J.-S.; Chun, K.S.; Joo, S.H. Thymoquinone induces apoptosis of human epi-dermoid carcinoma A431 cells through ROS-mediated suppression of STAT3. Chem. Biol. Interact. 2019, 312, 108799.
- Alam, M.F.; Khan, G.; Safhi, M.M.; Alshahrani, S.; Siddiqui, R.; Sivagurunathan, M.S.; Anwer, T. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice by modulating oxidative damage and cellular inflammation. Cardiol. Res. Pract. 2018, 2018, 1483041.
- Nagi, M.N.; Mansour, M.A. Protective effect of thymoquinone against doxorubicin–induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol. Res. 2000, 41, 283–289.
- Pehlivan, D.Y.; Durdağı, G. Effects of Thymoquinone on Blood Parameters in Doxorubicin Cardiotoxicity. Exp. Appl. Med. Sci. 2020, 1, 7–16.
- Karabulut, D.; Ozturk, E.; Kaymak, E.; Akin, A.T.; Yakan, B. Thymoquinone attenuates doxorubicin-cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22618.
- Nagi, M.N.; Al-Shabanah, O.A.; Hafez, M.M.; SayedAhmed, M.M. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011, 25, 135–142.
- Adalı, F.; Gonul, Y.; Kocak, A.; Yuksel, Y.; Ozkececi, G.; Ozdemir, C.; Tunay, K.; Bozkurt, M.F.; Sen, O.G. Effects of thymoquinone against cisplatin-induced cardiac injury in rats. Acta Cir. Bras. 2016, 4, 271–277.
- Dunn, B.K.; Wickerham, D.L.; Ford, L.G. Prevention of hormone-related cancers: Breast cancer. J. Clin. Oncol. 2005, 23, 357–367.
- Suddek, G.M. Protective role of thymoquinone against liver damage induced by tamoxifen in female rats. Can. J. Physiol. Pharmacol. 2014, 92, 640–644.
- Al-Malki, A.L.; Sayed, A.A. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC Complement. Altern. Med. 2014, 14, 282.
- Alenzi, F.Q.; El-Bolkiny, Y.E.; Salem, M.L. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br. J. Biomed. Sci. 2010, 67, 20–28.
- El-Sheikh, A.A.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat. Inflamm. 2015, 2015, 859383.
- Badary, O.A. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J. Ethnopharmacol. 1999, 67, 135–142.
- Elsherbiny, N.M.; El-Sherbiny, M. Thymoquinone attenuates Doxorubicin-inuced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem. Biol. Interact. 2014, 223, 102–108.
- El-Sheik, A.A.; Morsy, M.A.; Hamouda, A.H. Protective mechanisms of thymoquinone on methotrexate-induced intestinal toxicity in rats. Pharmacogn. Mag. 2016, 12, 76–81.
- Shahid, F.; Farooqui, Z.; Khan, A.A.; Khan, F. Oral Nigella sativa oil and thymoquinone administration ameliorates the effect of long-term cisplatin treatment on the enzymes of carbohydrate metabolism, brush border membrane and antioxidant defense in rat intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 145–157.
- Gore, P.R.; Prajapati, C.P.; Mahajan, U.B.; Goyal, S.N.; Belemkar, S.; Ojha, S.; Patil, C.R. Protective effect of thymoquinone against cyclophosphamide-induced hemorrhagic cystitis through inhibiting DNA damage and upregulation of Nrf2 expression. Int. J. Biol. Sci. 2016, 12, 944.
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104.
- Kirby, B.J.; Kopun, J.G.; Tan, H.; Neely, S.T.; Gorga, M.P. Do “optimal” conditions improve distortion product otoacoustic emission test performance? Ear Hear. 2011, 32, 230.
- Sagit, M.; Korkmaz, F.; Akcadag, A.; Somdas, M.A. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2231–2237.
- Gökçe, A.; Oktar, S.; Koc, A.; Yonden, Z. Protective effects of thymoquinone against methotrexate-induced testicular injury. Hum. Exp. Toxicol. 2011, 30, 897–903.
- Suddek, G.M.; Ashry, N.A.; Gameil, N.M. Thymoquinone attenuates cyclophosphamide-induced pulmonary injury in rats. Inflammopharmacology 2013, 21, 427–435.
- Ray, P.D.; Huang, B.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990.
- Kasapovi, J.; Stojiljkovi, V.; Todorovi, A.; Rado, L.; Sai, Z.S.; Pajovi, B. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5- fluorouracil, doxorubicin and cyclophosphamide. Clin Biochem. 2010, 43, 1287–1293.
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300.
- Prabasheela, B.; Singh, A.K.; Fathima, A.; Pragulbh, K.; Deka, N.J.; Kumar, R. RRST-Health Science Association between Antioxidant Enzymes and Breast Cancer. Recent Res. Sci. Technol. 2014, 3, 93–95.
- Available online: https://clinicaltrials.gov/ct2/results?term=thymoquinone (accessed on 20 November 2021).
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
07 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





