
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Taichi Ochi | + 1669 word(s) | 1669 | 2021-12-30 07:28:03 | | | |
| 2 | Jessie Wu | Meta information modification | 1669 | 2022-01-04 07:52:01 | | |
Video Upload Options
This entry describes that a new paradigm must be applied in which the relative value of antidepressant treatment is specifically weighted in terms of enabling the natural resilience process.
1. No Evidence for Specific (‘True’) and Nonspecific Antidepressant Drug Response
Recently, research about possible associations between the responses to antidepressant treatment and specific pharmacogenes in over 150 antidepressant-free patients with depression [1][2]. These newly admitted patients had not been treated with antidepressant drugs during the preceding six months and 54.5% had never been treated with antidepressant drugs during their entire life. They had a clinical diagnosis of depression according to ICD-10 criteria [3] of at least moderate severity, as measured by Hamilton’s depression rating scale (HAMD-17) [4][5], and were studied over four weeks. According to the old but still very influential theory of Quitkin et al. [6], activation of the ‘true’ antidepressant mechanism takes 2–4 weeks to occur after antidepressant therapy starts, the initial treatment response being comparable with that of the placebo, hence nonspecific or spontaneous. The existence of such a lag time indicates that the acute pharmacological effects occurring after a short time result in activation of a unified mechanism that alleviates the complete syndrome. With this theory in mind, we compared the response during the first two weeks with that during the last two weeks. For the total group, the average HAMD-17 score amounted to 24.3 ± 5.2 (mean ± standard deviation) at entry, and this decreased to 12.9 ± 4.9 and 5.0 ± 3.9 after treatment at two weeks and four weeks, respectively [1]. Obviously, in this study, there was no question of perceiving a delayed response of two to four weeks for the antidepressants to show an effect in the depressed patients. The indication of the existence of such a ‘lag time’ through clinical experience has been debated by several authors when considering the course of the response over time in different patient groups in meta-analysis of controlled clinical trials [7]. The results of the research group of the Zürich Psychiatric University Hospital are particularly convincing in this respect [7]. These authors studied the individual pattern of improvement in 2848 patients with MDD who had participated in four independent clinical trials using a total of seven different antidepressants and a placebo. They found that the period to the onset of improvement (the latency time) and the pattern of improvement did not differ between the verum treatment and the placebo; however, the number of responders (incidence of improvement) was higher with verum than with the placebo. The fact that the early and large response in Ochi et al.’s study [1] was related to a placebo effect (a spontaneous resilience mechanism) was contradicted by the results of another study on the same population by our research group, which investigated the influence of polymorphisms of the gene encoding for P-glycoprotein (ABCB1) on the timing of the observed improvement [2]. Certain genotypes caused a partial shift in the improvement during the first two weeks compared to the second two weeks of treatment. This indicates that a pharmacological effect may at least contribute to the improvement in the clinical condition. The transporter P-glycoprotein limits the passage of antidepressants through the blood–brain barrier, and certain genetic variants may influence the rapidity and intensity with which CNS structures are pharmacologically affected by antidepressants. Of note, Stassen et al. [7] suggested that antidepressants activate ‘a common, biological, ‘resilience’-like component that largely controls recovery from depression’.
2. A Theory of the Background Biopsychosocial Components
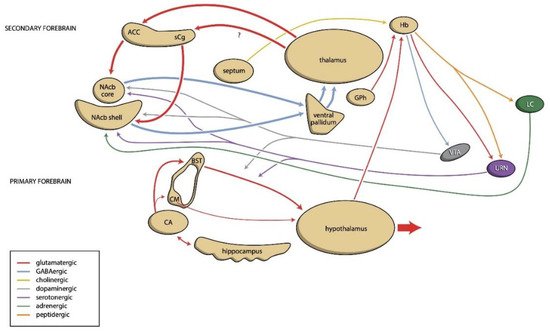
3. The Backgrounds of the Resilience Component
References
- Ochi, T.; Vyalova, N.M.; Losenkov, I.S.; Paderina, D.Z.; Pozhidaev, I.V.; Loonen, A.J.M.; Simutkin, G.G.; Bokhan, N.A.; Ivanova, S.A.; Wilffert, B. Limited Associations Between 5-HT Receptor Gene Polymorphisms and Treatment Response in Antidepressant Treatment-Free Patients with Depression. Front. Pharmacol. 2019, 10, 1462.
- Geers, L.M.; Ochi, T.; Vyalova, N.M.; Losenkov, I.S.; Paderina, D.Z.; Pozhidaev, I.V.; Simutkin, G.G.; Bokhan, N.A.; Wilffert, B.; Touw, D.J.; et al. Influence of eight ABCB1 polymorphisms on antidepressant response in treatment-free Russian patients with moderate or severe depression. Hum. Psychopharmacol. 2021, e2826.
- World Health Organization. International Statistical Classification of Diseases and Health Related Problems ICD-10; World Health Organization: Geneva, Switzerland, 2004.
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62.
- Williams, J.B.W.; Link, M.J.; Rosenthal, N.E.; Amira, L.; Terman, M. Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD); New York State Psychiatric Institute: New York, NY, USA, 1992.
- Quitkin, F.M.; Rabkin, J.G.; Ross, D.; Stewart, J.W. Identification of true drug response to antidepressants. Use of pattern analysis. Arch. Gen. Psychiatry 1984, 41, 782–786.
- Stassen, H.H.; Angst, J.; Hell, D.; Scharfetter, C.; Szegedi, A. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J. Clin. Psychiatry 2007, 68, 1195–1205.
- Loonen, A.J.M.; Ivanova, S.A. Circuits regulating pleasure and happiness: Evolution and role in mental disorders. Acta Neuropsychiatr. 2018, 30, 29–42.
- Loonen, A.J.M.; Ivanova, S.A. The evolutionary old forebrain as site of action to develop new psychotropic drugs. J. Psychopharmacol. 2018, 32, 1277–1285.
- Klein, R.G. Archeology and the evolution of human behavior. Evol. Anthropol. 2000, 9, 17–36.
- Vriezen, T.C.; Van der Woude, A.S. Job. In Oud-Israëlitische & Vroeg-Joodse Literatuur; Noort, E., Van der Sar, H.C., Van der Brom, L.J., Rouwhorst, G.A.M., Eds.; Kok: Kampen, The Netherlands, 2000; pp. 339–347.
- Ahonen, M. Ancient philosophers on mental illness. Hist. Psychiatry 2019, 30, 3–18.
- Daly, R.W. Before depression: The medieval vice of acedia. Psychiatry 2007, 70, 30–51.
- Safavi-Abbasi, S.; Brasiliense, L.B.; Workman, R.K.; Talley, M.C.; Feiz-Erfan, I.; Theodore, N.; Spetzler, R.F.; Preul, M.C. The fate of medical knowledge and the neurosciences during the time of Genghis Khan and the Mongolian Empire. Neurosurg Focus 2007, 23, E13.
- Jansson, Å. Mood disorders and the brain: Depression, melancholia, and the historiography of psychiatry. Med. Hist. 2011, 55, 393–399.
- Kraepelin, E. Psychiatrie. Ein Lehrbuch für Studierende und Ärzte, 8th ed.; Barth: Leipzig, Germany, 1913.
- Heckers, S.; Kendler, K.S. The evolution of Kraepelin’s nosological principles. World Psychiatry 2020, 19, 381–388.
- Van Bakel, A.H.A.C. The psychology of degeneration. An analysis of the theoretical foundations of Kraepelin’s nosology. Tijdschr. Voor Psychiatr. 1998, 40, 752–764.
- Hoff, P. The Kraepelinian tradition. Dialogues Clin. Neurosci. 2015, 17, 31–41.
- Shorter, E. The history of nosology and the rise of the Diagnostic and Statistical Manual of Mental Disorders. Dialogues Clin. Neurosci. 2015, 17, 59–67.
- Morozov, P.V.; Becker, R.A.; Bykov, Y.V. Titans of Psychiatry of the XX Century; Publishing House Gorodets: Moscow, Russia, 2020; p. 30.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013.
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision; 2018). Available online: https://icd.who.int/browse11/l-m/en (accessed on 25 October 2021).




