
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaehwan Kim | + 2026 word(s) | 2026 | 2021-04-19 08:20:44 | | | |
| 2 | Nora Tang | + 4 word(s) | 2030 | 2021-12-30 07:06:53 | | | | |
| 3 | Nora Tang | Meta information modification | 2030 | 2021-12-31 08:34:55 | | |
Video Upload Options
By increasing the environmental concerns and depletion of petroleum resources, bio-based resins have gained interest. Recently, lignin-based resins have attracted attention due to their low cost, environmental benefits, good thermal stability, excellent mechanical properties, and suitability for high-performance natural fiber composite applications. This content highlights the recent use of lignin-based resins with natural fiber composites for high-performance applications.
1. Introduction
Lignin is the second most abundant and high molecular weight natural phenolic polymer, which occurs from plant tissues. It contains a three-dimensional phenolic structure built up by p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol [1], as shown in Figure 1. Although the industrial sector produces a large amount of lignin, around 95% every year, it is burned, resulting in severe resource waste [2]. Lignin has been used in limited industrial applications due to the complex structure with amphiphilic nature. Lignin is commonly soluble in dimethyl sulfoxide, but it also soluble in some organic solvents and aqueous organic solvent solution [3][4]. Despite the complex structure, lignin has recently attracted interest in many applications due to its inherent potential from its unique behaviors, such as antioxidants in plastics, scavengers, surfactants, and dispersants antibacterial, antitumoral, and adhesives [5][6][7]. However, despite the broad use of lignin as a bio-based feedstock for materials, it shows incompatibility with other polymers and heterogeneity, leading to the deterioration of materials’ properties. Several studies have been reported to modify the lignin and enhance compatibility with polymers and mechanical properties for high-performance applications to overcome these drawbacks. Up to now, a large amount of research has been performed on bio-based thermoplastics such as bismaleimide/eugenol [8], poly(lactic acid) [9], bio-poly(ethylene terephthalate) [10], poly(butylene succinate) [11], polyhydroxyalkanoates [12][13], poly(ethylene 2,5-furandicarboxylate) [14], and also on thermosets such as itaconic acid [15], isosorbide [16], composite of cardanol novolac/bismaleimide [17], plant oil [18][19], eugenol [20], and polyesters [21]. On the other hand, average research progress has been published on preparing thermoplastic and thermosets resins with lignin. In engineering fields, thermoset resins are generally used in electronic components, adhesive, aircraft industry, and automotive because of their high strength, high modulus, good durability, good thermal stability, and chemical resistance [22][23]. Thermoset resins have a wide range of properties that depend on the curing cycle, hardener, and the proportions of hardener used during the preparation of thermoset resins [24].
2. History and Development
As a cross-linked natural polymer containing phenolic content and low-cost availability, Lignin has been studied for a broad range of applications [25][26]. It is produced from the pulp and paper industry around 70 million tons per year, but only 2% of it has been used to recover energy in the pulping industry, and the rest is uncommercialized waste materials [27]. As a bio-based material, it has been used in several applications. Nevertheless, owing to the decomposition of amorphous phenolic structure, higher char and tar formation during the conversion process, it is difficult to develop higher value materials [28]. Instead, the researcher’s attention has been increased due to lignin’s unique properties, such as low toxicity, biodegradability, ecofriendly, and sensitiveness to enzymatic degradation [29]. In recent years, lignin has been used with various polymers to prepare composites and epoxy resins for different applications, i.e., surfactants, coatings, lubricants, plasticizers, carbon fiber, wastewater treatment, stabilizing agents, and fire retardant [1][24][30][31][32].
Commonly, polymeric resins are categorized into epoxy and ester ones, in which ester resins are utilized for low-performance applications while epoxy resins for high performance [21][23][33][34]. Epoxy resins have more adhesion tendency to the surface of glass and carbon fibers than ester resins. Therefore, they are used for high-performance applications [35][36]. Compared to synthetic resins, recently, bio-based resins aim to develop thermosets, thermoplastic, and lightweight materials with better mechanical properties and replace commercial petroleum-based resins safe for environmental and healthcare concerns. Due to higher properties, bio-based resins’ demand is more increasing day by day than before [37][38][39][40]. Wood, a naturally higher strength fiber-reinforced composite, consists of lignin, cellulose, and hemicelluloses. Lignin plays the resin role as a waterproof and thermostability, while cellulose contributes as reinforcement [22]. Due to the thermostability and adhesive properties, the lignin-based resin has been suggested for fiber composite applications [41][22][42]. Several researchers have reported the lignin-based resin namely, hemp-epoxy [43], lignin/poly(ethylene oxide) [41][44], lignin/PVA [22][42], DL/epoxy [45], lignin/phenol formaldehyde [25][46], and fiber-reinforced composites [47]. Lignin-based resins have been used in broad applications such as sports equipment, tennis racquets, airplane, boats, and construction components for buildings due to their lightweight, high specific modulus and strength. Benjamin and coworkers [43] investigated the lignin weight percent effect into the hemp-epoxy composite to improve the fiber-to-matrix adhesion’s structural properties. The hemp-epoxy composite was developed by a vacuum-assisted resin transfer molding, in which epoxy was used as resin while hemp was used as reinforcement fiber. They found that 2.5 wt% of lignin is the optimized condition to improve Young’s modulus and ultimate tensile strength. Still, more than 2.5 wt% of lignin, these properties decreased due to the excessive portion of lignin particle that prevents the complete wetting from the fiber reinforcement. It was observed that the interfacial adhesion of 2.5 wt% lignin-based composite was better than those of 1.0 wt% lignin-based composite due to increasing the viscosity during the infusion.
On the other hand, the pull-out of the composite increased at 5.0 wt% lignin due to some porosity of composite [48]. The lignin hemp-epoxy composite is applicable for commercial products based on structural properties. Liu et al. [49] developed the lignin-based toughen epoxy resin (Scheme 1) and investigated alkali lignin carboxylic acid (AL-COOH) content effect on toughness reinforcement of the resin. At 1.0 wt% of AL-COOH, the resin's fracture toughness is better than the neat epoxy resin. The lignin epoxy resin exhibited a roughen fracture surface, but the neat epoxy had a smooth surface with some river-like lines. It concluded that the AL-COOH was incorporated into the epoxy resin at the molecular level. The toughened lignin epoxy resin is a bio-renewable candidate of coating material for high-performance composites.
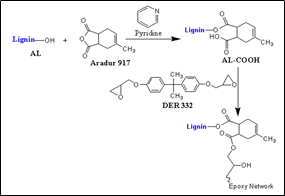
Scheme 1. An illustration reaction scheme of the lignin-based network.
Lignin-based epoxy composite is developed through the loading of DL-epoxy into the cured epoxy networks [45]. It was observed that DL-epoxy was successfully incorporated into the epoxy network in the form of dark dots. These dots occurred due to DL-epoxy’s aggregation, caused by strong π–π stacking and hydrogen bond interaction. The fracture toughness of DL-epoxy/cured epoxy composite was evaluated using a critical stress intensity factor (K1c). K1c increased with increasing the fraction of DL-epoxy in the composite. At 1.0 wt% of DL-epoxy fraction, the composite showed the highest K1c near to 1.78 MPa.m1/2. Conversely to neat epoxy, DL-epoxy/cured epoxy exhibited low Tg due to the low cross-linking density at 1.0 wt% of DL-epoxy fraction [50]. The toughness depends on the feed ratios, cross-linking density, and Tg [51][52][53]. As compared to the straight fracture lines of neat epoxy, DL-epoxy/cured epoxy composite showed an irregular fracture surface with a large surface area. It indicates that the higher energy consumption for crack shows a more ductile nature than neat epoxy. Overall, after the incorporation of DL-epoxy, the fracture toughness, tensile strength, and ductility improved. Besides, its approaches are useable for composites application as additives.
Ferdosian et al. studied lignin-based epoxy’s composites to investigate the flexural and adhesion strength properties. As compared to petroleum-based epoxy’s composites, lignin-based epoxy’s composites showed the lower flexural strength. In contrast, as increased the loading wt% of lignin-based epoxy matrix into the fiber-reinforced plastics, the composites demonstrated the nearest flexural modulus with petroleum-based matrix. Additionally, the adhesion strength of composites improved as the loading wt% of DOL-epoxy in the epoxy resin containing DGEBA increased. The loading 50 wt% of DOL-epoxy’ composites showed higher adhesion strength around 7.7 MPa (Table 1). The results of lignin-based epoxy resin indicated that it is a bio-based candidate for the high-performance application as a coating materials [54]. To this regard, Kraft lignin (KL) based resin was prepared by the cross-linking of glycerol diglycidyl ether (GDE) to reduce the utilization of formaldehyde-based resin. It can play a significant role as an adhesive at the industrial scale because it can be easily produced from various sources. The lignin-based adhesive was applied to the plywood to fabricate the composites and optimized the resin’s adhesion strength between plywood layers. The adhesion strength is increased with the increasing cross-linker ratios. At loading smaller particle size (>37 μm) of lignin in the resin, the resin showed better adhesion strength than higher particle size (>250–500 μm) of lignin. Note that the higher adhesion strength owing to the more cross-linking because smaller particles expose higher –OH groups for cross-linking. As compared to commercially based plywood samples that made by formaldehyde based resin, KL-GDE based resin depicted the better adhesion strength around 2.0–2.5 MPa (Table 1). The results indicates that KL-GDE resin’s performance is comparable to the formaldehyde-based resins [55].
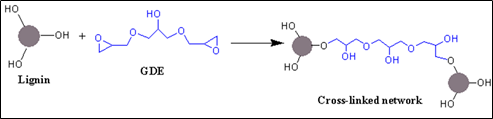
Scheme 2. An illustration reaction scheme of the cross-linked lignin-based network.
In this regard, Ko and co-workers [42] have reported the PVA-lignin resin as shown in Scheme 3, and its adhesive property and toughness to cellulose nanofiber (CNF) film was investigated by the lap-shear joint (LSJ) test (Figure 1a). 50 wt% PVA-lignin resins showed comparable properties with epoxy resin. After the LSJ test, PVA-lignin resin remained on both CNF films, which appeared due to the enhanced interaction between CNFs and PVA-lignin resin. It was observed that the shear strength increased with the increasing wt% of the PVA fraction. At 50 wt% PVA, PVA-lignin resin exhibited higher toughness (0.6MPa) with high shear strength (4.4 MPa) (Table 1). Figures 1b and 1c showed the cross-sectional SEM images of PVA-lignin and poly(ethylene oxide) (PEO)-lignin SLJ test specimens.
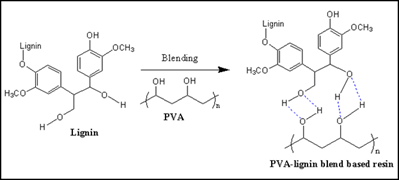
Scheme 3. Representative reaction scheme of PVA-lignin-based resin.
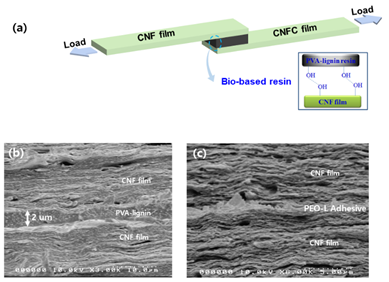
Figure 1. An illustration of the shear lap joint test of bio-based resins with CNF films (a) and cross-sectional SEM images of the specimens for PVA-lignin (b) [41] and PEO-lignin resins (c) [15].
Jayaramudu and co-workers [41] have prepared the PEO-lignin blend resin for the CNFs film, as shown in Scheme 4. They evaluated the effect of the lignin fraction on the blend's adhesive property by using the SLJ test. Relative to the shear strength of pure PEO (0.44 MPa), PEO-lignin resin exhibited a shear strength of 0.84 MPa at 30 wt% lignin content (Table 1). The SEM result demonstrated that the CNF films are jointed with PEO-lignin resin through hydrogen bonding (Figure 1c), and it could be used as an adhesive for fiber application.
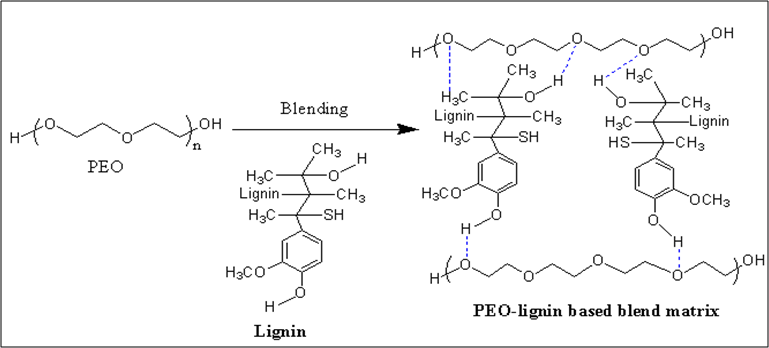
Scheme 4. Representative reaction scheme of PEO-lignin-based resin.
Recently, Ko et al. [22] have reported the esterified PVA-lignin-maleic acid (EPLM) resin (Scheme 5). The waterproof, toughness, and adhesive behavior of EPLM resin were investigated at various wt% of MA fraction. The water contact angle increased with the increasing wt% of the MA fraction, which indicates the hydrophobicity of resin. Note that the hydrophilicity nature of materials affects the adhesion properties because if resins have a hydrophilic nature, they can absorb moisture, resulting in the adhesion may deteriorate between the substrate surface and resins [56][57][58]. The failure strength of EPLM resin increased with the rising wt% of MA fraction, and it was found that the EPLM resin showed the highest failure strength (6.8 MPa) at 40 wt% of MA, whereas the failure strength for PVA-lignin resin is 4.4 MPa (Table 1). The higher toughness, hydrophobicity, and adhesive behaviors can be applied for natural fiber reinforcement.
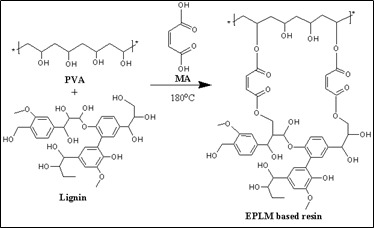
Scheme 5. Representative reaction scheme of EPLM-based resin.
Table 1. Adhesion strength performance of lignin-based resins.
|
Resin |
Substrate |
Shear strength (MPa) |
Ref. |
|
PVA-Lignin |
CNFs |
4.4 |
[41] |
|
PEO |
CNFs |
0.442 |
[42] |
|
30%PEO-L |
CNFs |
0.865 |
[42] |
|
EPL40 |
CNFs |
6.8 |
[23] |
|
50%DOL-DDM |
Stainless steel |
7.7±0.3 |
[54] |
|
KL-GDE |
Birch plywood |
2.1 |
[55] |
3. Summary
This entry elucidated broad research literature and synthetic activities on lignin-based resins, focusing majorly on adhesion property and mechanical properties. Although commercial epoxy resins are prevalent for various applications, bio-based epoxy resins from lignin could be alternates for commercial epoxy resins due to their higher properties with desirable eco-friendly and environmental safety. This entry explored the utilization of lignin bio-based resin to develop natural fiber/polymer composites with high-performance applications. With more attempts from the worldwide research community, advanced high-performance lignin-based epoxy resins might be a technical and commercial success for natural fiber composite applications. This entry will increase the research community’s attention to bio-based resins production to reduce the use of petroleum-based resins. The literature depicts limited research progress on resin’s adhesion strength with natural fibers, including natural fiber composites development with lignin-derived resins. However, this literature shows challenges that must be overcome to develop fully bio-based resins, including enhancing resin’s binding adhesion strength with natural fibers to obtain environment-safe high-performance composites.
References
- Li, J.; Wang, W.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins. RSC Adv. 2016, 6, 67435–67443.
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176.
- Dastpak, A.; Lourençon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility study of lignin in industrial organic solvents and investigation of electrochemical properties of spray-coated solutions. Ind. Crops Prod. 2020, 148, 112310.
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular Lignin Solubility and Structure in Organic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 17839–17850.
- Toh, K.; Yokoyama, H.; Noda, H.; Yuguchi, Y. Antioxidant capacity of lignin from green tea waste. J. Food Biochem. 2010, 34, 192–206.
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463.
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Applicability of lignins from different sources as antioxidants based on the protective effects on lipid peroxidation induced by oxygen radicals. Ind. Crops Prod. 2009, 30, 184–187.
- Shibata, M.; Teramoto, N.; Shimasaki, T.; Ogihara, M. High-performance bio-based bismaleimide resins using succinic acid and eugenol. Polym. J. 2011, 43, 916–922.
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262.
- Kint, D.; Muñoz-Guerra, S. A review on the potential biodegradability of poly(ethylene terephthalate). Polym. Int. 1999, 48, 346–352.
- Huang, X.; Li, C.; Zheng, L.; Zhang, D.; Guan, G.; Xiao, Y. Synthesis, characterization and properties of biodegradable poly(butylene succinate)-block-poly(propylene glycol)segmented copolyesters. Polym. Int. 2009, 58, 893–899.
- Woolnough, C.A.; Yee, L.H.; Charlton, T.; Foster, L.J.R. Environmental degradation and biofouling of “green” plastics including short and medium chain length polyhydroxyalkanoates. Polym. Int. 2010, 59, 658–667.
- Woolnough, C.A.; Charlton, T.; Yee, L.H.; Sarris, M.; Foster, L.J.R. Surface changes in polyhydroxyalkanoate films during biodegradation and biofouling. Polym. Int. 2008, 57, 1042–1051.
- Xie, H.; Wu, L.; Li, B.G.; Dubois, P. Modification of Poly(ethylene 2,5-furandicarboxylate) with Biobased 1,5-Pentanediol: Significantly Toughened Copolyesters Retaining High Tensile Strength and O2 Barrier Property. Biomacromolecules 2019, 20, 353–364.
- Ma, S.; Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254.
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783.
- Shibata, M.; Itakura, Y.; Watanabe, H. Bio-based thermosetting resins composed of cardanol novolac and bismaleimide. Polym. J. 2013, 45, 758–765.
- Jian, X.Y.; An, X.P.; Li, Y.D.; Chen, J.H.; Wang, M.; Zeng, J.B. All Plant Oil Derived Epoxy Thermosets with Excellent Comprehensive Properties. Macromolecules 2017, 50, 5729–5738.
- Kamjornsupamitr, T.; Hunt, A.J.; Supanchaiyamat, N. Development of hyperbranched crosslinkers from bio-derived platform molecules for the synthesis of epoxidised soybean oil based thermosets. RSC Adv. 2018, 8, 37267–37276.
- Chen, C.H.; Tung, S.H.; Jeng, R.J.; Abu-Omar, M.M.; Lin, C.H. A facile strategy to achieve fully bio-based epoxy thermosets from eugenol. Green Chem. 2019, 21, 4475–4488.
- Nameer, S.; Johansson, M. Fully bio-based aliphatic thermoset polyesters via self-catalyzed self-condensation of multifunctional epoxy monomers directly extracted from natural sources. J. Coat. Technol. Res. 2017, 14, 757–765.
- Ko, H.U.; Kim, J.W.; Kim, H.C.; Zhai, L.; Kim, J. Esterified PVA-lignin resin by maleic acid applicable for natural fiber reinforced composites. J. Appl. Polym. Sci. 2019, 137, 48836.
- Bobade, S.K.; Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Bio-Based Thermosetting Resins for Future Generation: A Review. Polym. Plast. Technol. Eng. 2016, 55, 1863–1896.
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901.
- Park, Y.; Doherty, W.O.S.; Halley, P.J. Developing lignin-based resin coatings and composites. Ind. Crops Prod. 2008, 27, 163–167.
- Rico-Garc, D.; Ruiz-Rubio, L.; Leyre, P. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81.
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019.
- Yang, L.; Seshan, K.; Li, Y. A review on thermal chemical reactions of lignin model compounds. Catal. Today 2017, 298, 276–297.
- Sawamura, K.; Tobimatsu, Y.; Kamitakahara, H.; Takano, T. Lignin Functionalization through Chemical Demethylation: Preparation and Tannin-Like Properties of Demethylated Guaiacyl-Type Synthetic Lignins. ACS Sustain. Chem. Eng. 2017, 5, 5424–5431.
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512.
- Liu, W.; Yao, Y.; Fu, O.; Jiang, S.; Fang, Y.; Wei, Y.; Lu, X. Lignin-derived carbon nanosheets for high-capacitance supercapacitors. RSC Adv. 2017, 7, 48537–48543.
- Kumari, S.; Chauhan, G.S.; Monga, S.; Kaushik, A.; Ahn, J.H. New lignin-based polyurethane foam for wastewater treatment. RSC Adv. 2016, 6, 77768–77776.
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable. Polymers 2017, 9, 523.
- Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110.
- Hernandez, D.A.; Soufen, C.A.; Orlandi, M.O.; Paulista, U.E.; Paulista, U.E. Carbon Fiber Reinforced Polymer and Epoxy Adhesive Tensile Test Failure Analysis Using Scanning Electron Microscopy. Mater. Res. 2017, 20, 951–961.
- Kwiecie, A.; Krajewski, P.; Hojdys, Ł.; Tekieli, M.; Słonski, M. Flexible Adhesive in Composite-to-Brick Strengthening—Experimental and Numerical Study. Polymers 2018, 10, 356.
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701.
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A Review. Polym. Int. 2018, 67, 815–839.
- Ma, S.; Li, T.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 66, 164–173.
- Heinrich, L.A.; Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888.
- Jayaramudu, T.; Ko, H.; Chan, H.; Woong, J.; Sik, E.; Kim, J. Adhesion properties of poly (ethylene oxide) -lignin blend for nanocellulose composites. Compos. Part B 2019, 156, 43–50.
- Ko, H.U.; Zhai, L.; Park, J.H.; Lee, J.Y.; Kim, D.; Kim, J. Poly(vinyl alcohol)–lignin blended resin for cellulose-based composites. J. Appl. Polym. Sci. 2018, 135, 46655.
- Wood, B.M.; Coles, S.R.; Maggs, S.; Meredith, J.; Kirwan, K. Use of lignin as a compatibiliser in hemp/epoxy composites. Compos. Sci. Technol. 2011, 71, 1804–1810.
- Yu, Q.; Bahi, A.; Ko, F. Influence of Poly (ethylene oxide) (PEO) Percent and Lignin Type on the Properties of Lignin /PEO Blend Filament. Macromol. Mater. Eng. 2015, 300, 1023–1032.
- Sun, J.; Wang, C.; Chee, J.; Yeo, C.; Yuan, D.; Li, H.; Stubbs, L.P.; He, C. Lignin Epoxy Composites: Preparation, Morphology, and Mechanical Properties. Macromol. Mater. Eng. 2016, 301, 328–336.
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of Wheat Straw Alkali Lignin for Application in Phenol Formaldehyde Adhesives. Polymers 2016, 8, 209.
- Nair, S.S.; Dartiailh, C.; Levin, D.B.; Yan, N. Highly Toughened and Transparent Biobased Epoxy Composites Reinforced with Cellulose Nanofibrils. Polymers 2019, 11, 612.
- Madsen, B.; Lilholt, H. Physical and mechanical properties of unidirectional plant fibre composites—An evaluation of the influence of porosity. Compos. Sci. Technol. 2003, 63, 1265–1272.
- Liu, W.; Zhou, R.; Li, H.; Goh, S.; Huang, S.; Lu, X. From Waste to Functional Additive: Toughening Epoxy Resin with Lignin. ACS Appl. Mater. Interfaces 2014, 6, 5810–5817.
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352.
- Robinson, E.J.; Douglas, E.P.; Mecholsky, J.J. The Effect of Stoichiometry on the Fracture Toughness of a Liquid Crystalline Epoxy. Polym. Eng. Sci. 2002, 42, 269–279.
- Ashrafi, B.; Martinez-rubi, Y.; Khoun, L.; Simard, B.; Johnston, A. Influence of the reaction stoichiometry on the mechanical and thermal properties of SWCNT-modified epoxy composites. Nanotechnology 2013, 26, 265701.
- Liu, T.; Nie, Y.; Chen, R.; Zhang, L.; Meng, Y.; Li, X. Hyperbranched polyether as an all-purpose epoxy modifier: Controlled synthesis and toughening mechanisms. J. Mater. Chem. A 2015, 3, 1188.
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C.C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165.
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466.
- Rohart, V.; Lebel, L.L.; Dubé, M. Effects of environmental conditions on the lap shear strength of resistance-welded carbon fibre/thermoplastic composite joints. Compos. Part B Eng. 2020, 198, 108239.
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976.
- Honda, K.; Morita, M.; Otsuka, H.; Takahara, A. Molecular aggregation structure and surface properties of poly(fluoroalkyl acrylate) thin films. Macromolecules 2005, 38, 5699–5705.




