Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hafiz Muhammad Adeel Sharif | + 729 word(s) | 729 | 2021-12-13 05:05:37 | | | |
| 2 | Nora Tang | Meta information modification | 729 | 2021-12-30 02:17:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharif, H.M.A. Low Temperature-Based Abatement Technique. Encyclopedia. Available online: https://encyclopedia.pub/entry/17638 (accessed on 07 February 2026).
Sharif HMA. Low Temperature-Based Abatement Technique. Encyclopedia. Available at: https://encyclopedia.pub/entry/17638. Accessed February 07, 2026.
Sharif, Hafiz Muhammad Adeel. "Low Temperature-Based Abatement Technique" Encyclopedia, https://encyclopedia.pub/entry/17638 (accessed February 07, 2026).
Sharif, H.M.A. (2021, December 30). Low Temperature-Based Abatement Technique. In Encyclopedia. https://encyclopedia.pub/entry/17638
Sharif, Hafiz Muhammad Adeel. "Low Temperature-Based Abatement Technique." Encyclopedia. Web. 30 December, 2021.
Copy Citation
Nitrogen and sulpher oxides (NOx, SOx) have become a global issue in recent years due to the fastest industrialization and urbanization. Numerous techniques are used to treat the harmful exhaust emissions, including dry, traditional wet and hybrid wet-scrubbing techniques. However, several difficulties, including high-energy requirement, limited scrubbing-liquid regeneration, formation of secondary pollutants and low efficiency, limit their industrial utilization. Regardless, the hybrid wet-scrubbing technology is gaining popularity due to low-costs, less-energy consumption and high-efficiency removal of air pollutants.
removal
wet-scrubbing
catalytic systems
1. Metal Ligand Absorption
Researchers and environmentalists have been working on alternative solutions to the concerns listed above and explored various approaches. Another critical thing to be discussed here is the NO solubility problem in water. Some other toxic gases such as CO2 and SO2 are easily treated via the alkali absorption method [1][2]. Since the solubility problem NO can be treated such as other gases, it required some combined or integrated system applied for NO reduction [3]. This solubility problem indicated the NOx removal would be easier if chemical modifications were used for its absorption and reduction. Many ways came to notice, but most practice has been undertaken by wet scrubbing using Fe-EDTA. There are several reasons for considering flue gas treatment and reduction techniques, which are discussed herein. The MnOx/CNTs catalysts were found to have unusual SCR activity at low temperatures when they were first synthesized. When using the optimal 1.2 percent MnOx/CNTs catalyst at 80–180 °C, the NO conversion ranged between 57.4 and 89.2 percent. This occurred from the use of amorphous MnOx catalysts, which have a higher ratio of Mn4+ to Mn3+ and OS to (OS + OL) than the crystalline MnOx catalysts [4]. By impregnation and in situ deposition methods, the same Ce/Mn molar ratio was achieved in the preparation of Ce (1.0) Mn/TiO2 catalysts. In comparison to the impregnation-prepared Ce(1.0)Mn/TiO2-IP catalyst, the in situ deposition-prepared Ce(1.0)Mn/TiO2-SP catalyst demonstrated superior catalytic activity throughout a wide temperature range (150–300 °C) and at high-gas hourly-spaced velocities ranging from 10,500 to 27,000 h−1. Furthermore, the Ce(1.0)Mn/TiO2-SP catalyst produced by the in situ deposition approach has superior sulphur resistance to the Ce(1.0)Mn/TiO2-IP catalyst [5][6]. By using the citric acid–ethanol dispersion method, a variety of Gadolinium (Gd)-modified MnOx/ZSM-5 catalysts were produced and assessed using a low-temperature NH3-SCR reaction. Of them, the GdMn/Z-0.3 catalyst, which had a molar ratio of 0.3 for Gd to Mn, had the maximum catalytic activity, and it was capable of achieving a 100 percent NO conversion in the temperature range of 120–240 degrees Celsius. Furthermore, when tested in the presence of 100 ppm SO2, GdMn/Z-0.3 demonstrated superior SO2 resistance when compared to Mn/Z. It was demonstrated that such catalytic efficacy was primarily driven by surface chemisorbed oxygen species, a wide surface area, an abundance of Mn4+ and, a proper acidity and reducibility, and the of the catalyst, among other factors [7].
2. Metal Ligand Stability
Among transition metal chelating complexes, Fe-EDTA is the most favorable and stable chelate against the long-range of pH. Comparison for the metal chelate system are also shown in Figure 3. On top of that, ferrous (Fe(II)) has the great affinity towards the NO compared to other d-block elements. By taking advantage of this Fe-EDTA stability and greatest affinity, it has been used for NOx removal from flue gas. Another advantage of this process (wet scrubbing) is the simultaneous removal of NOx and SO2 under ambient conditions. Moreover, the NOx interaction with Fe(II) is direct chemically binding and forms a very stable bond among other transition metals [8][9].
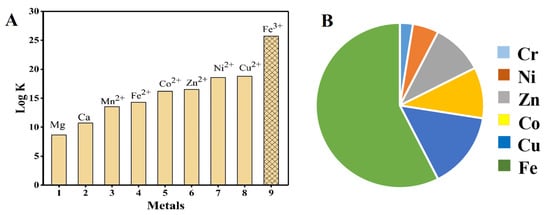
Figure 3. (A) Relative stability of Fe-EDTA with other metals, (B) relative NO gas and transition metal interactions.
3. Principle of Gas Absorption
Basically, this concept is composed of two steps, (i) Fe-EDTA solution absorbs NOx molecule via making metal-nitrosyl-complex and then (ii) this NOx reduced into N2O, N2 and N-S compounds by utilizing the SO2, which is a compulsory part of flue gas. This SO2 transformed into SO32− by alkali absorption and reduced NOx by converting itself into SO42− ions. During the reduction of NOx, the scrubber (Fe-EDTA) also regenerated irrespective the valence form of iron, Fe(II) or Fe(III). As Fe(III) is more stable due to more stability than Fe(II), it cannot be restored without the help of the electron donor externally. The number of electron donors used to reduce Fe(III) back to Fe(II) depends upon the source, condition and more important the NOx reduction process.
References
- Zhao, C.; Chen, X.; Zhao, C. CO2 Absorption Using Dry Potassium-Based Sorbents with Different Supports. Energy Fuels 2009, 23, 4683–4687.
- Chang, J.C.S.; Kaplan, N. SO2 removal by limestone dual alkali. Environ. Prog. 1984, 3, 267–274.
- Barman, S.; Philip, L. Integrated System for the Treatment of Oxides of Nitrogen from Flue Gases. Environ. Sci. Technol. 2006, 40, 1035–1041.
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths from COVID-19. JAMA 2021, 325, 133–134.
- Zhang, X.; Zhang, X.; Yang, X.; Chen, Y.; Hu, X.; Wu, X. CeMn/TiO2 catalysts prepared by different methods for enhanced low-temperature NH3-SCR catalytic performance. Chem. Eng. Sci. 2021, 238, 116588.
- Djellabi, R.; Yang, B.; Xiao, K.; Gong, Y.; Cao, D.; Sharif, H.M.A.; Zhao, X.; Zhu, C.; Zhang, J. Unravelling the mechanistic role of Ti-O-C bonding bridge at titania/lignocellulosic biomass interface for Cr(VI) photoreduction under visible light. J. Colloid Interface Sci. 2019, 553, 409–417.
- Guan, J.; Zhou, L.; Li, W.; Hu, D.; Wen, J.; Huang, B. Improving the Performance of Gd Addition on Catalytic Activity and SO2 Resistance over MnOx/ZSM-5 Catalysts for Low-Temperature NH3-SCR. Catalysts 2021, 11, 324.
- Ramachandran, B.; Herman, R.G.; Choi, S.; Stenger, H.G.; Lyman, C.E.; Sale, J.W. Testing zeolite SCR catalysts under protocol conditions for NOx abatement from stationary emission sources. Catal. Today 2000, 55, 281–290.
- Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 290–309.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
595
Revisions:
2 times
(View History)
Update Date:
30 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




