
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keith Stine | + 3294 word(s) | 3294 | 2021-11-29 09:01:41 | | | |
| 2 | Bruce Ren | Meta information modification | 3294 | 2021-12-14 02:32:05 | | |
Video Upload Options
The fundamental essence of material design lies in bringing together the competing aspects of a large specific surface area and rapid transport pathways. This review summarizes the recent advances in the strategies to create a hierarchical bicontinuous morphology in porous metals, focusing mainly on the hierarchical architectures in nanoporous gold. Understanding the advantages of generating hierarchical structures on distinct and well-defined length scales can play a huge role in solving problems in porous materials and can guide the synthesis of new materials for specific applications.
1. Introduction
The study of the corrosion properties of alloys has added substantial value in the field of scientific study that deals with the aspects of electrochemical kinetics with morphological evolution of surfaces along with the contributions from ancient history on depletion gilding. Depletion Gilding or Mise en Couleur is the technique of changing the surface composition of an alloy by the removal of the less noble metal from the surface layer giving rise to a surface-enriched product. The pre-Columbian populations of South America mastered the art of “Depletion Gilding” on an alloy of gold (Au) and copper (Cu) by selective corrosion of copper in acid media. It is pertinent to review the historical evidence of this 2000-year-old process used for surface enrichment due to its relevance to some of the more modern processes that will be discussed in the later sections [1][2][3][4]. The 1980s marked the beginning of an era when considerable insight was put into understanding the micromorphological changes occurring near the surface after alloy corrosion. Corrosion tunnels in Au-Cu alloys were seen for the first time by Pickering and Swann using transmission electron microscopy. It was found that closely spaced pits or tunnels appeared to be the dominant features that were dependent on the alloy composition and the nature of the corroding reagent [5]. Through the pioneering work by Forty and Pickering, the process of dealloying and the unusual appearance of an open bicontinuous nanoporous microstructure was understood. Recently, Erlebacher et al. explained the atomistic details regarding the generation of porosity during dealloying. His model was based on “interfacial phase separation” in which he explained the formation of clusters and islands of gold rather than uniform spreading over the surface, which in turn passivated the interface and stopped further etching. Combining the knowledge gained from the historical connection between dealloying and depletion gilding, he fabricated ultra-thin nanoporous gold membranes having a large surface area [6].
Nanoporous metals have attracted much attention owing to their extraordinary electrical and optical properties due to the unique bicontinuous interpenetrating ligaments and open pores present in their structural architecture [7]. Two different nanofabrication methods for creating novel functional materials are classified as bottom-up and top-down approaches. Specifically, top-down approaches create nanoscale structures by the controlled removal of materials from bulk solid or by adding patterns on a blank canvas of the bulk sample. In contrast, the bottom-up approach involves the self-assembly of precursors into the final structure of the desired arrangement. Fine-tuning of features at the microscale and larger length scales in an arbitrary fashion can be achieved using top-down approaches while researchers can modulate molecular-scale lengths using bottom-up methods of synthesis [8]. However, bulk nanoporous metals suffer from transport limitations where diffusion-based mass transport is slow through the bicontinuous network. This limits their benefits in the field of catalysis, sensors, actuators, and chemical separations. To resolve the aforementioned issue, the best approach will be the addition of a structural hierarchy incorporating microporous transport in the entire structure, which also ensures the accessibility to the large surface area of the porous structure [9].
Hierarchical organization is most evident in biological systems such as the porous network seen in bones and trees, wherein the ordered hierarchy reduces the density of the structure, provides channels for easy flow of fluids and nutrients, and enhances mechanical properties. To mimic such advanced systems where structural elements themselves have a structure on a smaller scale requires the appropriate preparation procedures along with the impact of introducing such hierarchy on the functional aspect of the material [10]. Over the last decade, a range of strategies has been employed to fabricate hierarchically porous materials, which include surfactants as soft templates to create materials with dual mesoporous structures [11], hard template method [12], supercritical fluid technology [13], freeze-drying [14], self-assembly approach using metal alkoxides [15], etc.
There is widespread interest in the development of hierarchically porous materials owing to their diversity and performance. Figure 1 shows the major areas where hierarchically designed materials can be employed for better sensitivity and selectivity.
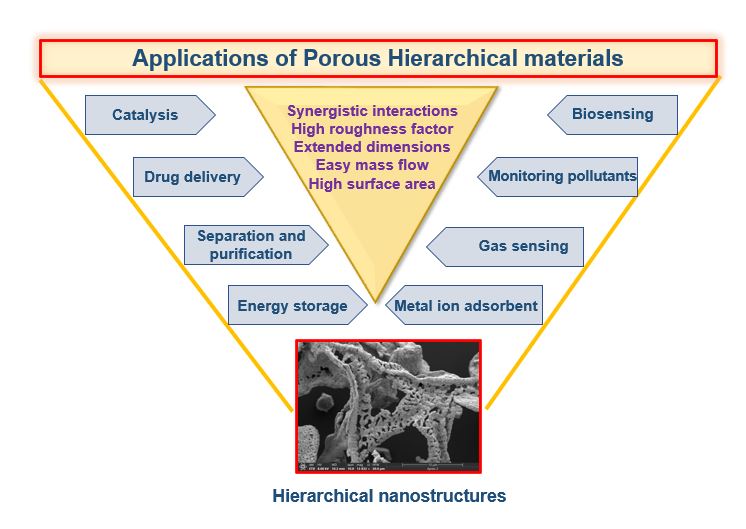
Figure 1. Schematic showing the wide-ranging areas involving the use of porous hierarchical materials.
In this review, we will describe some of the most prominent approaches used to create structural hierarchy in nanoporous gold along with some other coinage metals. We hope that with this review the reader will gain in-depth knowledge of designing and controlling a specific pore structure along with the prospects associated with such materials.
2. Methods to Generate the Hierarchy
There have been tremendous efforts devoted to developing new approaches to functional hierarchical nanostructures, which are of both scientific and technological importance. The simple dealloying method has been known for years for creating functional nanoporous metals with tunable structural features. In particular, NPG prepared using alloy corrosion exhibits a series of intriguing properties to be used successfully in catalysis, sensing, and surface plasmon resonance. Considering the great success of the method that has been traditionally regarded as a destructive process, research efforts are focused on extending the dealloying methodology encompassing other advanced technologies [16]. This paper will summarize some of the general approaches in developing a hierarchical material at various length scales. Figure 2 depicts the top-down and bottom-up approaches in creating hierarchy in material design.
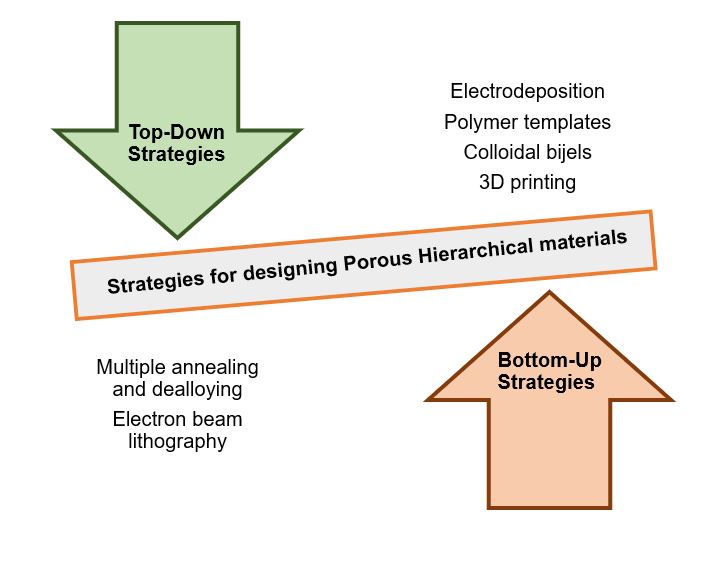
Figure 2. Diagram showing the common methodologies under the top-down and bottom-up approaches.
2.1. Multiple Annealing and Dealloying
Researchers have long endeavored to create hierarchical porous metallic materials using dealloying with or without their combination with other manufacturing processes. It has been seen that the dealloying method has the potential to create a structural hierarchy for promising applications [17][18]. Hierarchical nanoporous materials provide a large surface area and a structure rich in pore channels. They can be created by utilizing the dealloying approach that depends on the chemical stability difference of the constituent elements in the starting alloy material [19]. One-step dealloying is an attractive route to fabricate hierarchical nanoporous structures. Hierarchical nanoporous Cu@Cu2O composite was easily fabricated using dealloying of CuAl thin foils in dilute NaOH solution until no hydrogen bubbles were generated. Selective dissolution of Al from CuAl alloy gave rise to a sponge-like structure with multimodal pore size distributions. The catalyst prepared in this way has shown advanced performance towards one-pot transfer hydrogenation reactions of diverse nitroarenes to anilines [20]. NPG with a bimodal pore size distribution has been created using a multistep process involving corrosion-coarsening-Ag plating-realloying-corrosion. The selective dissolution of silver from silver/gold alloy was done by immersing it in nitric acid for 1 h. The remaining gold rearranges into the 3D porous network, which was annealed at an elevated temperature of 400 °C for 8 h for expanding the pore size to create an upper hierarchy. Pores of this annealed membrane were filled with silver using the gas-phase electroless plating technique and the second annealing step was performed to rehomogenize the material. The second dealloying step dissolved the remaining silver generating a hierarchical porous network. This diffusion-based process was applied to 100 nm thin, porous membranes leading to large porosity channels and small porosity channel walls with tunable pore size [21]. Hierarchically porous Au-Cu alloy film was created following a similar series of alloying/dealloying processes. The fabrication process involved electrodeposition of copper on a substrate followed by annealing and selective dissolution of copper from the alloy. The resulting architecture is comprised of micrometer-sized ligament channels and small ligament pores in the nanometer range. The catalytic activity for methanol electro-oxidation in alkaline solution using such materials has shown their remarkable performance in the field of catalysis and sensing [22]. Recently, an electrochemical dealloying strategy has been used to create “nested-network” nanoporous gold (N3PG) bulk samples. Dilute alloys of Au in Ag were arc melted and homogenized at 850 °C. Cuboid samples created from the ingots were dealloyed under 300 mV potential control. An alloy sample with high silver content was used to ensure enough silver content is remaining for a second dealloying step. The first dealloying step created a ligament size of ~16 nm, which upon annealing at 300 °C for 3 h coarsened to ~200 nm. A second dealloying at 750 mV created an architecture consisting of a fine network of nanoscale ligaments nested within the upper hierarchy network [23].
2.2. Electrochemical Approach
Electrodeposition is a powerful technique and a convenient tool to create nanostructures by controlling the growth rate via altering the deposition potential [24]. One such surfactant and template-free route to create hierarchical dendritic gold microstructures (HDGMs) with secondary and tertiary branches have been used in the past using constant potential electrolysis at −0.6 V in 0.1 M Na2SO4 and 30 mM HAuCl4 serving as the electrolyte against indium tin oxide as the working electrode and platinum wire and saturated calomel electrode used as counter and reference electrode, respectively [25]. To generate structural hierarchy, nanoporous metals can be used as a coating on preexisting porous structures. Multimodal porosity is generated by sputtering or electrodeposition of a binary alloy on a 3D microporous template and then finally dealloying it to generate a nanoporous film [26]. Using this approach, an alloy film of Au-Sn has been galvanostatically electrodeposited on nickel foam in the alloy plating solution with a current density of 5 A dm−2 for 10 min. Selective etching of Sn was performed by immersing the sample into a 5 M NaOH and 1 M H2O2 solution for three days leading to the formation of 3D hierarchical porous NPG/Ni foam. The open and porous structure facilitates mass transport and charge transfer, which may hold great potential for its use in electrode material for electrocatalytic reduction of peroxide and other electrochemical reactions [27].
2.3. Synthetic and Natural Templates
Two approaches that can control the porosity within metals are dealloying and templating. Multimodal porous noble metals have been synthesized using a combination of techniques involving templating, slipcasting, and dealloying. Hierarchically porous gold monolith has been prepared using the dual approach of templating and dealloying on polystyrene (PS) beads working as the templates. Ag/Au PS core-shell particles were prepared using the electroless deposition approach with control over the sequence of metal deposition. The mole ratio of Au:Ag was adjusted during the plating process followed by casting the beads and heating them to remove the PS template. Hollow shells of Au/Ag alloy were placed in dilute nitric acid for dealloying [28]. Another route involving double-templating has also been used in the past to create highly ordered macro/mesoporous hierarchical metal architectures. It involves the electrodeposition of Au/Ag alloy within the void spaces of PS microspheres, which are closely packed within the micropores of a polycarbonate membrane. Dealloying and template dissolution gave rise to a highly regular 3D hierarchical gold structure with tunable morphology and porosity. The new double-templated electrodeposition approach is very attractive as the final material holds considerable promise for designing electrocatalytic surfaces for enhanced oxygen reduction reaction and hydrogen-peroxide detection [29]. Hierarchically porous gold structures have also been prepared by taking advantage of the unique structures, morphologies, composition, and spatial organization of biological materials. The bio-templated strategy is a unique way of creating nanostructures in an ordered array. Multiple hierarchies have been created using fine structures present in grapefruit exocarp [30], pollen and Lepidopteran wings [31], and butterfly wing scales [32]. A relatively new class of soft materials known as bicontinuous interfacially jammed emulsion gels or bijels have been used in material synthesis platforms to fabricate next-generation gold electrodes. Bijels are complex fluids in which interpenetrating, continuous domains of two immiscible liquids are maintained by the colloidal particles that sequester to the fluid interface [33]. A novel synthetic route to fabricate bicontinuous hierarchical NPG monolith using colloidal bijel templates and a combination of nanocasting and chemical dealloying has been introduced very recently. The experimental design is simple without the use of sophisticated lab equipment. In this, bijels of 2,6-lutidine/water stabilized by colloidal silica microspheres were prepared. Further selective polymerization of lutidine-rich phase and draining of water-rich phase gave rise to continuous macropores along with textural pores of submicrometer scale within the cross-linked polymer phase. Polymerized bijels were then impregnated with silver and gold salt solutions and annealed to decompose the precursor, remove the polymer template, and create a homogeneous alloy system. The alloyed monolith created can be dealloyed traditionally using nitric acid to generate a hierarchical structure. It is believed that the use of bijels for creating hierarchically porous metal electrodes can offer enhanced performance for their use in supercapacitors, rechargeable batteries, and catalysis [34].
2.4. Additive Manufacturing Techniques
Research interest in the field of additive manufacturing (AM) is growing rapidly due to the flexibility in complex and custom design that the technique offers. In contrast to the traditional subtractive fabrication strategies, AM builds 3D structures layer by layer, utilizing the necessary amount of material and scaffolding. Introduced by Charles Hull in 1986, the process of stereolithography helped to enhance the area of material fabrication [35]. Additive manufacturing is a collection of techniques for the fabrication of 3D materials by computer-controlled sequential release of energy and/or material to specified points in space. Currently, AM techniques for metals are paving the way to design complex geometries with tailored mechanical properties [36]. Recently, 3D printed hierarchical gold samples were prepared involving a multistep procedure of printing, annealing, and dealloying. In this, a viscous paste-like ink of Au: Ag (30:70 atomic ratio) was prepared by mixing silver and gold clay composed of organic binder into an organic solvent for 1 min in a centrifugal mixer. For printing, the ink was loaded in a syringe barrel affixed with a micronozzle, and 3D architectures were created under computer numerical control. To homogenize the as-printed structures, the assembly was annealed at 850 °C for 12 h in air. Thermal decomposition of the polymer binder created microscale porosity, whereas nanoscale pores were created by dealloying Ag by placing the annealed sample in concentrated nitric acid [9]. Recently, a novel approach to fabricate sub-micron NPG disks and microscale NPG patterns by the combination of top-down lithography and bottom-up atomic dealloying has been used. The fabrication process involved the deposition of an adhesion layer consisting of 5 nm of Cr and 100 nm Au onto a silicon surface, followed by depositing a 90 nm alloy layer and a 20 nm Cr top layer. Patterning was done on poly (methyl methacrylate) (PMMA), which was spun onto the Cr top layer by exposing it to the SEM equipped with a nanopattern generation system. Pre-patterning of thin alloy films before time-controlled dealloying in nitric acid resulted in a hierarchical NPG structure. In situ patterning was achieved by restricting the reaction to only progress in-plane. The hierarchical NPG dot arrays generated via this methodology are effective for SERS and metal-enhanced fluorescence (MEF) techniques for highly sensitive molecular sensing [37].
2.5. Kirkendall Effect: Self Templated Methodology
The most widely used approaches to fabricate nanoporous hierarchical gold structures include dealloying, templating, and electrochemical synthesis. There is still scope to scale up the production of the fabricated material and eliminate the post-treatment steps leading to structural deformation, with more efficient and innovative procedures [38][39]. To overcome these limitations, a unique self-templated synthetic strategy based on the metallurgical concept of the Kirkendall effect has recently emerged, which explains the formation of voids at the interface of two metals due to their different interdiffusion rates. In nanochemistry, this effect is explained by the outward elemental diffusion leading to material flux across the interface and the formation of a series of void structures affected by reaction temperature and time [40]. The Kirkendall effect was first introduced in 1947 to generate pores in alloys. However, the methodology was not accepted by many due to the impairment of mechanical properties of the alloy [41][42]. Recently, synthesis of Kirkendall effect-based hollow 3D structures was achieved. In this work, investigations to use this effect systematically in the creation of 3D multilevel porous metal catalysts with tunable micropores ranging from 1.9–8.3 μm have been done. Synthesis was performed in a three-step strategy starting with controlled electrodeposition of Cu thin film at −1.8 V on 3D nickel foams using CuSO4 and boracic acid as the electrolyte. The Ni-Cu composite foams generated were further annealed at 1000–1100 °C and were finally electrochemically etched at 0.6 V, giving rise to dense arrays of micropores uniformly distributed on the microstruts of the foam. An array of applications in energy storage and conversion devices can make use of the unique properties of such materials [43]. Chemists have exploited the Kirkendall effect to prepare nanomaterials of unusual morphologies. There are several interesting reports regarding the fabrication of hierarchical porous transition metal oxide films utilizing the Kirkendall effect. One such report explained the hierarchical architecture in porous iron oxide films through a hydrothermal reaction between an iron substrate and iodine solution. Different morphologies were seen in different solvents owing to different reaction-diffusion rates of iodine with iron [44]. A series of novel hierarchical nanoporous microstructures have been synthesized wherein the Kirkendall effect is believed to facilitate the formation of pores.
3. Conclusions and Outlook
Recent progress made in the fabrication of hierarchical nanoporous gold and other metals has been reviewed. Every synthetic method was unique in creating a structural hierarchy with different pore dimensions and structures. However, the designing of porous materials with controllable ordered pore structures, crystalline framework, and structural stability still needs to be resolved for their practical applications. More progress has been made in the templated synthesis strategies or in employing a porogen concept to create hierarchical metals and composites [45]. They are most popular due to their flexibility and ability to precisely control the structural features via directing reactions to a certain region. It is still a great challenge to explore a simple, green, and low-cost route to fabricate hierarchical gold nanoporous structures with a “clean” surface. The issue may be addressed in the future by taking inspiration from nature for the development of newer and greener strategies. Green design and technology can mitigate negative impacts on the environment. Promising green materials including plants, fungi, microorganisms, enzymes, and biopolymers could be used in the future for creating hierarchy in porous metals. Another interesting research direction involves the use of functionalized ionic liquids in aqueous solutions for the controlled synthesis of various gold hierarchical architectures.
The development of 3D printing technology in medicine is proceeding very rapidly. Additionally, in the era of the digital world, artificial intelligence is emerging as a game-changer in healthcare systems. To gain benefits in the mainstream clinical practice, 3D printing harnessing modern technology of AI could potentially increase the performance by reducing the risk of error, ensuring stringent quality control, reducing material wastage, and giving the benefit of automated production [46][47]. Recently, AI has been utilized in designing novel materials with complex architectures and unique material properties such as elasticity, plasticity, and wear performance. The key issues lie in the selection of proper methodology and the positioning of the functional elements in the resulting 3D structure. However, with the integration of AI and material design, the study of bioinspired hierarchical materials has been done by casting the natural process of evolution into a computational framework and thereby accelerating the material property prediction process [48]. In the near future, it will be interesting to link the field of artificial intelligence with 3D printing technology to discover new microstructural patterns leading to advanced materials in a vast design space.
It is reasonable to expect that further modifications in the synthesis scheme to control the individual porosities and intricate morphologies may open a new arena for these materials. It is expected that this work will provide useful ideas for the future synthesis of hierarchical nanoporous gold and other metals to be used in electrochemical applications.
References
- Sparavigna, A.C. Depletion gilding: An ancient method for surface enrichment of gold alloys. Mech. Mater. Sci. Eng. 2016, 2, 1–8.
- Biener, J.; Biener, M.M.; Madix, R.J.; Friend, C.M. Nanoporous gold: Understanding the origin of the reactivity of a 21st century catalyst made by pre-columbian technology. ACS Catal. 2015, 5, 6263–6270.
- Lechtman, H. Pre-Columbian surface metallurgy. Sci. Am. 1984, 250, 56–63.
- Newman, R.C.; Corcoran, S.G.; Erlebacher, J.; Aziz, M.J.; Sieradzki, K. Alloy corrosion. MRS Bull. 2013, 24, 24–28.
- Forty, A.J. Micromorphological studies of the corrosion of gold alloys. Gold Bull. 1981, 14, 25–35.
- Ding, Y.; Kim, Y.-J.; Erlebacher, J. Nanoporous gold leaf: “Ancient technology”/advanced material. Adv. Mater. 2004, 16, 1897–1900.
- Qiu, H.J.; Li, X.; Xu, H.-T.; Zhang, H.-J.; Wang, Y. Nanoporous metal as a platform for electrochemical and optical sensing. J. Mater. Chem. C 2014, 2, 9788–9799.
- Isaacoff, B.P.; Brown, K.A. Progress in top-down control of bottom-up assembly. Nano Lett. 2017, 17, 6508–6510
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Worsley, M.A.; Ye, J.; Duoss, E.B.; Spadaccini, C.M.; et al. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459.
- Juarez, T.; Biener, J.; Weissmüller, J.; Hodge, A.M. Nanoporous metals with structural hierarchy: A review. Adv. Eng. Mater. 2017, 19, 1700389.
- Ali, D.; Zeiger, C.R.; Azim, M.M.; Lein, H.L.; Mathisen, K. Evaluation of surfactant templates for one-pot hydrothermal synthesis of hierarchical SAPO-5. Microporous Mesoporous Mater. 2020, 306, 110364.
- Wu, L.; Li, Y.; Fu, Z.; Su, B.-L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701.
- Castaño, M.; Martinez-Campos, E.; Pintado-Sierra, M.; García, C.; Reinecke, H.; Gallardo, A.; Rodriguez-Hernandez, J.; Elvira, C. Combining breath figures and supercritical fluids to obtain porous polymer scaffolds. ACS Omega 2018, 3, 12593–12599.
- Li, Y.; Liu, Q.; Kang, D.; Gu, J.; Zhang, W.; Zhang, D. Freeze-drying assisted synthesis of hierarchical porous carbons for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21016–21022.
- Yang, X.-Y.; Léonard, A.; Lemaire, A.; Tian, G.; Su, B.-L. Self-formation phenomenon to hierarchically structured porous materials: Design, synthesis, formation mechanism and applications. Chem. Commun. 2011, 47, 2763–2786.
- Xu, C.; Wang, R.; Chen, M.; Zhang, Y.; Ding, Y. Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246.
- Song, T.; Yan, M.; Qian, M. The enabling role of dealloying in the creation of specific hierarchical porous metal structures—A review. Corros. Sci. 2018, 134, 78–98.
- Chen, Q. Morphology Evolution in Dealloying. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, May 2013.
- Qiu, H.J.; Ito, Y.; Chen, M.W. Hierarchical nanoporous nickel alloy as three-dimensional electrodes for high-efficiency energy storage. Scr. Mater. 2014, 89, 69–72.
- Du, J.; Hou, J.; Li, B.; Qin, R.; Xu, C.; Liu, H. Support-free 3D hierarchical nanoporous Cu@Cu2O for fast tandem ammonia borane dehydrogenation and nitroarenes hydrogenation under mild conditions. J. Alloys Compd. 2020, 815, 152372.
- Ding, Y.; Erlebacher, J. Nanoporous metals with controlled multimodal pore size distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773.
- Xing, X.-F.; Han, D.-Q.; Wu, Y.-F.; Guan, Y.; Bao, N.; Xu, X.-H. Fabrication and electrochemical property of hierarchically porous Au-Cu films. Mater. Lett. 2012, 71, 108–110.
- Qi, Z.; Weissmüller, J. Hierarchical nested-network nanostructure by dealloying. ACS Nano 2013, 7, 5948–5954.
- DeMeo, D.; Macnaughton, S.; Sonkusale, S.; Vandervelde, T. Electrodeposited copper oxide and zinc oxide core-shell nanowire photovoltaic cells. In Nanowires—Implementations and Applications; IntechOpen: London, UK, 2011; pp. 141–156.
- Ye, W.; Yan, J.; Ye, Q.; Zhou, F. Template-free and direct electrochemical deposition of hierarchical dendritic gold microstructures: Growth and their multiple applications. J. Phys. Chem. C 2010, 114, 15617–15624.
- Sattayasamitsathit, S.; O’Mahony, A.M.; Xiao, X.; Brozik, S.M.; Washburn, C.M.; Wheeler, D.R.; Gao, W.; Minteer, S.; Cha, J.; Burckel, D.B.; et al. Highly ordered tailored three-dimensional hierarchical nano/microporous gold–carbon architectures. J. Mater. Chem. 2012, 22, 11950–11956.
- Li, Z.; He, Y.; Ke, X.; Gan, L.; Zhao, J.; Cui, G.; Wu, G. Three-dimensional nanoporous gold–cobalt oxide electrode for high-performance electroreduction of hydrogen peroxide in alkaline medium. J. Power Sources 2015, 294, 136–140.
- Nyce, G.W.; Hayes, J.R.; Hamza, A.V.; Satcher, J.H. Synthesis and characterization of hierarchical porous gold materials. Chem. Mater. 2007, 19, 344–346.
- Sattayasamitsathit, S.; Gu, Y.; Kaufmann, K.; Minteer, S.; Polsky, R.; Wang, J. Tunable hierarchical macro/mesoporous gold microwires fabricated by dual-templating and dealloying processes. Nanoscale 2013, 5, 7849–7854.
- Zhang, C.; Wang, J.; Hu, R.; Qiao, Q.; Li, X. Synthesis and gas sensing properties of porous hierarchical SnO2 by grapefruit exocarp biotemplate. Sens. Actuators B Chem. 2016, 222, 1134–1143.
- Wu, L.; He, J.; Shang, W.; Deng, T.; Gu, J.; Su, H.; Liu, Q.; Zhang, W.; Zhang, D. Optical functional materials inspired by biology. Adv. Opt. Mater. 2016, 4, 195–224.
- Tan, Y.; Gu, J.; Zang, X.; Xu, W.; Shi, K.; Xu, L.; Zhang, D. Versatile fabrication of intact three-dimensional metallic butterfly wing scales with hierarchical sub-micrometer structures. Angew. Chem. Int. Ed. 2011, 50, 8307–8311.
- Cates, M.E.; Clegg, P.S. Bijels: A new class of soft materials. Soft Matter 2008, 4, 2132–2138.
- Lee, M.N.; Santiago-Cordoba, M.A.; Hamilton, C.E.; Subbaiyan, N.K.; Duque, J.G.; Obrey, K.A.D. Developing monolithic nanoporous gold with hierarchical bicontinuity using colloidal bijels. J. Phys. Chem. Lett. 2014, 5, 809–812.
- Lee, C.-Y.; Taylor, A.C.; Nattestad, A.; Beirne, S.; Wallace, G.G. 3D printing for electrocatalytic applications. Joule 2019, 3, 1835–1849.
- Maleksaeedi, S.; Wang, J.K.; El-Hajje, A.; Harb, L.; Guneta, V.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Toward 3D printed bioactive titanium scaffolds with bimodal pore size distribution for bone ingrowth. Procedia CIRP 2013, 5, 158–163.
- Zhao, F.; Zeng, J.; Santos, G.M.; Shih, W.-C. In situ patterning of hierarchical nanoporous gold structures by in-plane dealloying. Mater. Sci. Eng.: B 2015, 194, 34–40.
- Ma, A.; Xu, J.; Zhang, X.; Zhang, B.; Wang, D.; Xu, H. Interfacial nanodroplets guided construction of hierarchical Au, Au-Pt and Au-Pd particles as excellent catalysts. Sci. Rep. 2014, 4, 4849.
- An, K.; Hyeon, T. Synthesis and biomedical applications of hollow nanostructures. Nano Today 2009, 4, 359–373
- Trogadas, P.; Ramani, V.; Strasser, P.; Fuller, T.F.; Coppens, M.-O. Hierarchically structured nanomaterials for electrochemical energy conversion. Angew. Chem. Int. Ed. 2016, 55, 122–148.
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714.
- Cao, S.; Zhang, Y.; Zhang, D.; Fan, J.; Zhang, J.; Zhou, J.; Zhang, J. Micrometer-scale Kirkendall effect in the formation of high-temperature-resistant Cr2O3/Al2O3 solid solution hollow fibers. Chem. Mater. 2018, 30, 5978–5986.
- Guo, J.; Chan, A.; Li, W.; Fan, D.L. Kirkendall effect in creating three-dimensional metal catalysts for hierarchically porous ultrathin graphite with unique properties. Chem. Mater. 2017, 29, 4991–4998.
- Zhang, L.; Yu, J.C.; Zheng, Z.; Leung, C.W. Fabrication of hierarchical porous iron oxide films utilizing the Kirkendall effect. Chem. Commun. 2005, 21, 2683–2685.
- Yang, X.-Y.; Li, Y.; Lemaire, A.; Yu, J.-G.; Su, B.-L. Hierarchically structured functional materials: Synthesis strategies for multimodal porous networks. Pure Appl. Chem. 2009, 81, 2265–2307.
- Banerjee, A.; Haridas, H.K.; SenGupta, A.; Jabalia, N. Artificial intelligence in 3D printing: A revolution in health care. In Emerging Applications of 3D Printing During COVID 19 Pandemic; Sandhu, K., Singh, S., Prakash, C., Sharma, N.R., Subburaj, K., Eds.; Springer: Singapore, 2022; pp. 57–79.
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol. Sci. 2021, 42, 745–757.
- Gu, G.X.; Chen, C.-T.; Richmond, D.J.; Buehler, M.J. Bioinspired hierarchical composite design using machine learning: Simulation, additive manufacturing, and experiment. Mater. Horiz. 2018, 5, 939–945.




