
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | BENIAMIN Oskar GRABAREK | + 1651 word(s) | 1651 | 2021-12-01 08:36:45 | | | |
| 2 | Amina Yu | Meta information modification | 1651 | 2021-12-14 03:58:59 | | |
Video Upload Options
Statins are one of the key drugs used in the course of hypercholesterolemia, the first of which is lovastatin, which was introduced to the market in 1987. Nowadays, mainly simvastatin, rosuvastatin and atorvastatin are used. The oldest and best-studied of them is simvastatin, but rosuvastatin is characterized by the greatest potency in the percentage reduction in low density lipoprotein (LDL), so it should be selected as the lipid-lowering drug of first choice in patients with cardiovascular diseases.
1. Introduction
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known as statins, are a commonly used and well-tolerated class of drugs used in lipid disorders, especially in cases of hypercholesterolemia. Their effectiveness in preventing the development of cardiovascular diseases makes statins one of the most widely used drugs [1]. While there are clear benefits of statins in reducing mortality in high-risk patients, it is still uncertain whether statins may increase or decrease the risk of cancer [2]. The effect of long-term statin use is quite complex, as they have multiple properties that go beyond lipid lowering. There is evidence that statins exacerbate endothelial dysfunction and decrease levels of inflammatory markers [3][4]; however, it is still unclear whether they influence cancer risk. The studies conducted so far have shown, on the one hand, the carcinogenic effect of statins [5], and, on the other hand, no effect on carcinogenesis [6] or even their protective effect [7][8].
There are currently many observational studies available that aim to assess the risk of individual cancers in long-term statin users. However, only some studies have found that statin use has lasted longer than 5 years [9]. None of these findings are statistically significant, except in one study showing a reduced risk of prostate cancer in 42 statin users [10]. In addition, it has been shown that over five years of statin use in 10 common cancers, including prostate, breast, colorectal, lung, bladder, pancreatic, renal cell, melanoma, endometrial cancer and non-Hodgkin lymphoma, significantly reduced the risk of hematological cancers and increased endometrial cancer risk associated with more than 5 years of use of statins [11].
2. Cholesterol Biosynthesis, Statin Structure and Their Applications
It has been shown that taking lipophilic statins such as simvastatin, atorvastatin, lovastatin, fluvastatin and pitavastatin, which can penetrate into cancer cells, is associated with a better prognosis in patients with breast cancer. On the other hand, improved survival in patients with hepatocellular carcinoma, colon cancer or prostate cancer is visible after the use of any statin [12]. The broad spectrum of the action of statins includes their effects on the proliferation, migration and survival of cancer cells by regulating the Rho factor, resistance to audiogenic seizures (Ras) and Rac-like guanosine triphosphate (GTP)-binding protein 1 (Rac) proteins, but also by inhibiting cancer cell growth modulated by specific pathways [13]. Co-administration of fluvastatin and vorinostat successfully induced apoptosis and reduced renal cancer growth in vitro and in vivo through the activation of 5’ adenosine monophosphate-activated protein kinase (AMPK), histone acetylation and cell stress induction [14]. Another study proved that simvastatin deregulated mutant p53 protein, activating the caspase-dependent apoptotic pathway and thus reducing the mobility of p53-mutated lung cancer cells [15]. In addition to their angiostatic effect, statins inhibit the synthesis of cytokines, including interleukin (IL-) IL-1β, IL-6, IL-8 and tumor necrosis factor alpha (TNF-α). The set of cytokines in the cluster of cancer cells stimulates them to proliferate and reorganize the spatial network of blood vessels, which promotes metastasis [16][17]. In studies conducted in mice transplanted with human breast cancer cells (MDA-MB-237), administration of cerivastatin to the tumor tissue significantly reduced the cross-linking of blood vessels of pathological tissue [18].
The integrity and permeability of blood vessels play an important role in metastasis. Statins have been shown to induce the expression of endothelial cadherins (including VE-cadherin), which are responsible for tight intercellular junctions. Increasing the number of cadherins is associated with strengthening vessels tightness, which limits the expansion of cancer cells in the body [19][20]. Moreover, statins inhibit the reprogramming of cancer cells into cancer stem cells responsible for metastasis and tumor immunity by reducing the transcriptional activity of the Yes1-associated transcriptional regulator (YAP1) and fafazzi (TAZ) and inhibiting geranylgeranyl pyrophosphate (GGPP) production [8].
Statins also regulate autophagy, which is triggered as a cellular defense mechanism when the elimination of toxic stress-inducing components or elements can ensure cell survival. It has been shown that autophagy can participate in both tumor suppression and its progression. On the one hand, it reduces the viability of cancer cells, is extremely important in the early stages of cancer promotion and represents a critical mechanism, and on the other hand, it stimulates aging processes caused by oncogenes. The tumor promoting effect of autophagy is mainly exerted in the later stages of cancer development. The dynamic proliferation of cancer cells requires large energy resources, which autophagy provides by recycling cell substrates [21]. Moreover, it promotes the survival of cancer cells under stressful conditions, such as tumor hypoxia and nutrient deficiency [22]. Simvastatin-induced autophagy has been reported in rhabdomyosarcoma cells [23]. It should be emphasized that autophagy also plays an important role in inducing resistance to chemotherapy [24].
An interesting phenomenon is that statins can also induce ferroptosis, which is another type of programmed cell death. This process is triggered by metabolic changes, and it is characterized by iron overload, accumulation of reactive oxygen species and lipid peroxidation, which can be used in statin therapy of cancer cells resistant to chemotherapy [24]. Another process induced by statins is pyroptosis, accompanied by cell swelling, rupture, lysis and, as a result, the release of pro-inflammatory cytokines. Studies have shown that pyroptosis is closely related to the development of various diseases, including cancer. Pyroptosis-inducing molecule gasdermin D (GSDMD) may be a promoter of gastric cancer cell proliferation, which has been proven both in in vitro and in vivo studies [25]. The potential use of statin-induced pyroptosis and its importance in the anticancer aspect requires further research and explanation. The potential mechanisms of the action of statins are shown in Figure 1 .
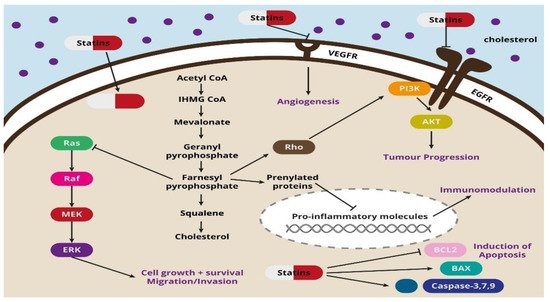
3. Cancer In Vitro Data and Statins
The Increased proliferation of cancer cells is caused by elevated cholesterol levels in these cells [26]. In vitro studies on medulloblastoma lines showed that simvastatin inhibits HMC-CoA reductase, thereby affecting the inhibition of metalloproteinase secretion via GGPP [8][27]. It is worth adding that HMG-CoA reductase is extremely important in maintaining cell viability, and its inhibition induces apoptosis and negatively affects cell proliferation. GGPP can serve as a substrate for protein prenylation or as a precursor for the synthesis of other metabolites, such as coenzyme Q or dolichols. In recent years, it has become clear that different types of cancer cells are dependent on GGPP synthesis [28]. Significant changes in the MMP-9 levels were observed in in vitro cultured human breast cancer cells [12]. Fluvastatin and simvastatin also reduced the release of MMP-9 in human and murine macrophages [29][30]. In turn, in studies of human microvascular endothelial cells (HMEC-1), cerivastatin dose-dependently reduced or completely blocked the expression or synthesis of MMP-2 [31].
In vitro pro-apoptotic activity has been documented in studies on cancer cell lines, including cancer of the cervix, pancreas, thyroid gland, prostate, colon, breast and larynx, as well as multiple myeloma or leukemia [20][21][22][23][24][25][32][33][34]. Importantly, statins activated various molecular events that promote apoptosis, depending on the type of cancer cell. Statins also affects the expression and activity of cyclins and cyclin-dependent kinases (CDKs) and their inhibitors. This leads to a blockage of the cell cycle at two of its critical points: G1/S and G2/M, thus inhibiting cell division. Under conditions where the statin dose is adequate and there is an imbalance between pro- and anti-apoptotic proteins, statins induce apoptosis [35][36][37][38][39][40][41][42].
One of the key mechanisms of the antitumor activity of statins is also the angiostatin effect in neoplastic lesions [43][44]. It is based on the expression modulation of anti- and pro-angiogenic factors, including VEGF, FGF and IGF-1, leading to the inhibition of angiogenesis and, as a result, reduction in metastatic capacity of cancer cells [43][45][46]. The dualistic nature of statins in terms of their role in the formation of blood vessels is observed in scientific literature, where the vast majority of studies report the anti-angiogenic effects of statins on cancer cells; however, a few demonstrate their ability to stimulate endothelial cell division and angiogenesis [19][44][47].
The clear presence of metalloproteinases in tumor masses, through the remodeling of extracellular matrix and basement membrane, has an unfavorable prognosis [48]. Organizational changes in the cell cytoskeleton, destabilization of actin filaments or changes in morphology favoring apoptosis are also a consequence of statins’ influence on cancer cells. The great potential of statins in terms of their use as drugs delaying progression and expansion of cancer has been proven in the course of experimental work [12][27][49].
4. Animal Cancer In Vivo Data and Statins
Studies have shown that statins, in this case simvastatin, induce the phosphorylation of endothelial nitric oxide synthase, inhibiting apoptosis and promoting Akt-dependent angiogenesis in the ischemic limbs of normocholesterolemic rabbits [50]. Lipophilic statins have higher cytotoxic potential and are more pro-apoptotic than hydrophilic statins, and therefore may be more beneficial in cancer treatment [46].
In in vivo studies in mice, pitavastatin was injected intraperitoneally, which inhibited the growth of subcutaneous glioma cells [51]. Simvastatin tested in a mouse model in which a human lung cancer xenograft was introduced inhibited tumor growth and bone metastasis, which occurred with a simultaneous reduction in MAPK/ERK activity [36]. The inhibition and delay of tumor growth was also observed in a pancreatic cancer xenograft in mice treated with a combination of gemcitabine and fluvastatin [52]. Advanced clinical trials are ongoing, with a large amount of promising in vitro and in vivo data on the efficacy of statin therapy in several different cancer models [8][53].
References
- Ingersgaard, M.V.; Helms Andersen, T.; Norgaard, O.; Grabowski, D.; Olesen, K. Reasons for Nonadherence to Statins—A Systematic Review of Reviews. Patient Prefer. Adherence 2020, 14, 675–691.
- Brugts, J.J.; Yetgin, T.; Hoeks, S.E.; Gotto, A.M.; Shepherd, J.; Westendorp, R.G.; de Craen, A.J.; Knopp, R.H.; Nakamura, H.; Ridker, P.; et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ 2009, 338, b2376.
- Ruszkowski, P.; Masajtis-Zagajewska, A.; Nowicki, M. Effects of combined statin and ACE inhibitor therapy on endothelial function and blood pressure in essential hypertension—A randomised double-blind, placebo controlled crossover study. J. Renin. Angiotensin. Aldosterone Syst. 2019, 20, 1470320319868890.
- Sahebkar, A.; Kiaie, N.; Gorabi, A.M.; Mannarino, M.R.; Bianconi, V.; Jamialahmadi, T.; Pirro, M.; Banach, M. A comprehensive review on the lipid and pleiotropic effects of pitavastatin. Prog. Lipid Res. 2021, 84, 101127.
- Zhou, Y.Y.; Zhu, G.Q.; Wang, Y.; Zheng, J.N.; Ruan, L.Y.; Cheng, Z.; Hu, B.; Fu, S.W.; Zheng, M.H. Systematic review with network meta-analysis: Statins and risk of hepatocellular carcinoma. Oncotarget 2016, 7, 21753–21762.
- Lubet, R.A.; Boring, D.; Steele, V.E.; Ruppert, J.M.; Juliana, M.M.; Grubbs, C.J. Lack of efficacy of the statins atorvastatin and lovastatin in rodent mammary carcinogenesis. Cancer Prev. Res. 2009, 2, 161–167.
- Zaleska, M.; Mozenska, O.; Bil, J. Statins use and cancer: An update. Future Oncol. 2018, 14, 1497–1509.
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic effects of statins: A focus on cancer. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165968.
- Boudreau, D.M.; Yu, O.; Johnson, J. Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 2010, 9, 603–621.
- Flick, E.D.; Habel, L.A.; Chan, K.A.; Van Den Eeden, S.K.; Quinn, V.P.; Haque, R.; Orav, E.J.; Seeger, J.D.; Sadler, M.C.; Quesenberry, C.P.; et al. Statin Use and Risk of Prostate Cancer in the California Men’s Health Study Cohort. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2218–2225.
- Jacobs, E.J.; Newton, C.C.; Thun, M.J.; Gapstur, S.M. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011, 71, 1763–1771.
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144.
- Fatehi Hassanabad, A. Current perspectives on statins as potential anti-cancer therapeutics: Clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res. 2019, 8, 692–699.
- Okubo, K.; Isono, M.; Miyai, K.; Asano, T.; Sato, A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2020, 111, 112–126.
- Chou, C.-W.; Lin, C.-H.; Hsiao, T.-H.; Lo, C.-C.; Hsieh, C.-Y.; Huang, C.-C.; Sher, Y.-P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403.
- Solimando, A.G.; Summa, S.D.; Vacca, A.; Ribatti, D. Cancer-Associated Angiogenesis: The Endothelial Cell as a Checkpoint for Immunological Patrolling. Cancers 2020, 12, 3380.
- Stockmann, C.; Schadendorf, D.; Klose, R.; Helfrich, I. The Impact of the Immune System on Tumor: Angiogenesis and Vascular Remodeling. Front. Oncol. 2014, 4, 69.
- Denoyelle, C.; Albanese, P.; Uzan, G.; Hong, L.; Vannier, J.P.; Soria, J.; Soria, C. Molecular mechanism of the anti-cancer activity of cerivastatin, an inhibitor of HMG-CoA reductase, on aggressive human breast cancer cells. Cell. Signal. 2003, 15, 327–338.
- Khaidakov, M.; Wang, W.; Khan, J.A.; Kang, B.-Y.; Hermonat, P.L.; Mehta, J.L. Statins and angiogenesis: Is it about connections? Biochem. Biophys. Res. Commun. 2009, 387, 543–547.
- Al Thawadi, H.; Abu-Kaoud, N.; Al Farsi, H.; Hoarau-Véchot, J.; Rafii, S.; Rafii, A.; Pasquier, J. VE-cadherin cleavage by ovarian cancer microparticles induces β-catenin phosphorylation in endothelial cells. Oncotarget 2016, 7, 5289–5305.
- Kenific, C.M.; Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015, 25, 37–45.
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64.
- Araki, M.; Motojima, K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem. Biophys. Res. Commun. 2008, 367, 462–467.
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 1–33.
- Wang, W.J.; Chen, D.; Jiang, M.Z.; XU, B.; Li, X.W.; Chu, Y.; Zhang, Y.J.; Mao, R.; Liang, J.; Fan, D.M. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J. Dig. Dis. 2018, 19, 74–83.
- Sławińska, A.; Kandefer-Szerszeń, M. The anticancer properties of statins. Postepy Hig. Med. Dosw. (Online) 2008, 62, 393–404.
- Sheikholeslami, K.; Ali Sher, A.; Lockman, S.; Kroft, D.; Ganjibakhsh, M.; Nejati-Koshki, K.; Shojaei, S.; Ghavami, S.; Rastegar, M. Simvastatin Induces Apoptosis in Medulloblastoma Brain Tumor Cells via Mevalonate Cascade Prenylation Substrates. Cancers 2019, 11, 994.
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as Anticancer Agents in the Era of Precision Medicine. Clin. Cancer Res. 2020, 26, 5791–5800.
- Mach, F. Statins as immunomodulators. Transpl. Immunol. 2002, 9, 197–200.
- Lev, S.; Gilburd, B.; Lahat, N.; Shoenfeld, Y. Prevention of tumor spread by matrix metalloproteinase-9 inhibition: Old drugs, new concept. Eur. J. Intern. Med. 2002, 13, 101–103.
- Vincent, L.; Soria, C.; Mirshahi, F.; Opolon, P.; Mishal, Z.; Vannier, J.-P.; Soria, J.; Hong, L. Cerivastatin, an Inhibitor of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase, Inhibits Endothelial Cell Proliferation Induced by Angiogenic Factors In Vitro and Angiogenesis in In Vivo Models. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 623–629.
- Harvey, Z.H.; Chen, Y.; Jarosz, D.F. Protein-Based Inheritance: Epigenetics beyond the Chromosome. Mol. Cell 2018, 69, 195–202.
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505.
- Xie, H.J.; Noh, J.H.; Kim, J.K.; Jung, K.H.; Eun, J.W.; Bae, H.J.; Kim, M.G.; Chang, Y.G.; Lee, J.Y.; Park, H.; et al. HDAC1 Inactivation Induces Mitotic Defect and Caspase-Independent Autophagic Cell Death in Liver Cancer. PLoS ONE 2012, 7, e34265.
- Crescencio, M.E.; Rodríguez, E.; Páez, A.; Masso, F.A.; Montaño, L.F.; López-Marure, R. Statins Inhibit the Proliferation and Induce Cell Death of Human Papilloma Virus Positive and Negative Cervical Cancer Cells. Int. J. Biomed. Sci. 2009, 5, 411.
- Zhao, J.; Xu, C.; Yao, J.; Yu, C.; Liao, L.; Dong, J. Statins and Thyroid Carcinoma: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 47, 1422–1431.
- Babcook, M.A.; Joshi, A.; Montellano, J.A.; Shankar, E.; Gupta, S. Statin Use in Prostate Cancer: An Update. Nutr. Metab. Insights 2016, 9, 43.
- Oechsle, C.M.; Showalter, L.E.; Novak, C.M.; Czerniecki, B.J.; Koski, G.K. Statin Drugs Plus Th1 Cytokines Potentiate Apoptosis and Ras Delocalization in Human Breast Cancer Lines and Combine with Dendritic Cell-Based Immunotherapy to Suppress Tumor Growth in a Mouse Model of HER-2pos Disease. Vaccines 2020, 8, 72.
- Alkhatib, M.H.; Al-Merabi, S.S. In vitro assessment of the anticancer activity of simvastatin-loaded microemulsion in liver and colon cancer cells. J. Drug Deliv. Sci. Technol. 2014, 24, 373–379.
- Tsubaki, M.; Fujiwara, D.; Takeda, T.; Kino, T.; Tomonari, Y.; Itoh, T.; Imano, M.; Satou, T.; Sakaguchi, K.; Nishida, S. The sensitivity of head and neck carcinoma cells to statins is related to the expression of their Ras expression status, and statin-induced apoptosis is mediated via suppression of the Ras/ERK and Ras/mTOR pathways. Clin. Exp. Pharmacol. Physiol. 2017, 44, 222–234.
- Burke, L.P.; Kukoly, C.A. Statins induce lethal effects in acute myeloblastic lymphoma cells within 72 hours. Leuk. Lymphoma 2008, 49, 322.
- Deng, D.H. Statins are Potential Anticancer Agents for Multiple Myeloma. Biomed. J. Sci. Tech. Res. 2019, 20, 14761–14762.
- Llevadot, J.; Asahara, T. Efecto de las estatinas en la inducción de angiogénesis y vasculogénesis. Rev. Española Cardiol. 2002, 55, 838–844.
- Dulak, J.; Jozkowicz, A. Anti-Angiogenic and Anti-Inflammatory Effects of Statins: Relevance to Anti-Cancer Therapy. Curr. Cancer Drug Targets 2005, 5, 579–594.
- Shiota, M.; Hikita, Y.; Kawamoto, Y.; Kusakabe, H.; Tanaka, M.; Izumi, Y.; Nakao, T.; Miura, K.; Funae, Y.; Iwao, H. Pravastatin-induced proangiogenic effects depend upon extracellular FGF-2. J. Cell. Mol. Med. 2012, 16, 2001–2009.
- Jang, H.J.; Hong, E.M.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; Lee, J. Statin induces apoptosis of human colon cancer cells and downregulation of insulin-like growth factor 1 receptor via proapoptotic ERK activation. Oncol. Lett. 2016, 12, 250–256.
- Skaletz-Rorowski, A.; Walsh, K. Statin therapy and angiogenesis. Curr. Opin. Lipidol. 2003, 14, 599–603.
- Stryjkowska-Góra, A.; Karczmarek-Borowska, B.; Góra, T.; Krawczak, K. Statins and cancers. Współczesna Onkol. 2015, 3, 167–175.
- Altwairgi, A.K. Statins are potential anticancerous agents (Review). Oncol. Rep. 2015, 33, 1019–1039.
- Kureishi, Y.; Luo, Z.; Shiojima, I.; Bialik, A.; Fulton, D.; Lefer, D.J.; Sessa, W.C.; Walsh, K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000, 6, 1004–1010.
- Jiang, P.; Mukthavaram, R.; Chao, Y.; Nomura, N.; Bharati, I.S.; Fogal, V.; Pastorino, S.; Teng, D.; Cong, X.; Pingle, S.C.; et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br. J. Cancer 2014, 111, 1562–1571.
- Liu, H.; Wang, Z.; Li, Y.; Li, W.; Chen, Y. Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma 2013, 60, 240–246.
- Bocci, G.; Fioravanti, A.; Orlandi, P.; Bernardini, N.; Collecchi, P.; Del Tacca, M.; Danesi, R. Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells. Br. J. Cancer 2005, 93, 319–330.




