Preparations containing calcipotriol combined with betamethasone dipropionate (in the forms of ointment, gel, and foam) are available for the topical treatment of psoriasis. It has been documented that foams provide higher bioavailability, resulting in increased efficacy in plaque psoriasis compared to ointments and gels. Gels or foams are preferred by patients for their different practical qualities (e.g., gels for “easy application”, and foams for “immediate relief”). The available data indicate that ointments may be the most effective formulation in nail psoriasis, and gels are preferred by patients with scalp psoriasis because of their cosmetic features. Treatment with a foam formulation is associated with a lower number of medical appointments compared to treatment with an ointment and with a lower probability of developing indications for systemic treatment. The safety profiles of foams, ointments, and gels are comparable, with the most common adverse effect being pruritus at the application site (in 5.8% of the patients). A long-term proactive maintenance therapy markedly reduces the number of relapses and is likely to close the gap between topical and systemic treatment in psoriasis.
1. Pharmacodynamics of Calcipotriol/Betamethasone Dipropionate
Calcipotriol, a synthetic vitamin D3 analogue, has a similar mode of action to calcitriol—changing the expression of genes responsive to vitamin D. It binds to the retinoid X receptor and influences cell differentiation and growth regulation, immune functions, and the balance of calcium and phosphorus in the body
[1]. It also has a reductive effect on the hyperproliferation of keratinocytes, normalizes their differentiation, and reduces the pro-inflammatory cytokine level, which induces anti-inflammatory and immunomodulatory effects
[1][2].
Betamethasone dipropionate belongs to the group of synthetic fluorinated glucocorticoids that exhibit anti-inflammatory and immunosuppressive effects by binding to glucocorticoid cytosolic receptors and then translocating to the nucleus where they regulate the transcription of numerous genes responsible for the immune response. It limits inflammatory infiltration, erythema, and edema, inhibits cell hyperproliferation, and improves the differentiation of keratinocytes in psoriasis
[2][3].
Pharmacodynamic studies showed the anti-inflammatory and immunoregulatory synergy of the combination of calcipotriol and betamethasone dipropionate with respect to the effects of these active substances administered individually
[4]. The effectiveness of calcipotriol/betamethasone dipropionate mixtures is related to a synergy of action of the two substances. Calcipotriol affects keratinocyte differentiation, while betamethasone influences inflammatory processes and minimizes skin irritation (e.g., pruritus) after calcipotriol application
[5].
The mechanism of calcipotriol/betamethasoneantipsoriatic activity has remained only partially known for a long time. However, in the last decade, a number of publications have started to discuss the immune background of psoriasis and the influence of T cells, B cells, dendric cells, as well as cytokines in its pathogenesis
[6][7][8][9]. A novel approach to the investigation of the mechanism of action of therapeutics applied in psoriasis treatment has been proposed. Recently, Satake et al.
[10] have investigated the synergistic effects of drug substances in combination therapy with Cal/BS for dermatitis-like psoriasis. They investigated the basic immune mechanisms in a mouse model of imiquimod-induced psoriasis. Cal/BS combination appeared effective in inhibiting the effects induced by imiquimod in comparison with a monotherapy with calcipotriol or betamethasone. The authors emphasized that Cal/BS synergistically induced CD8
+ regulatory T cells and improved the balance between CD8
+ or CD4
+ regulatory T cells and pro-inflammatory CCR6
+ γδ T17 lymphocytes in the lymph nodes. The data indicated that the synergistic antipsoriatic effect of Cal/BS was based on a reduction of the imbalance between regulatory CD8
+ or CD4
+ T cells and pro-inflammatory CCR6
+ γδ T17 cells.
Calcipotriol is stable in alkaline solutions with pH above 8, whereas betamethasone dipropionate requires an acidic environment with pH between 4 and 6. Therefore, the presence of both substances in an aqueous environment leads to interactions and to their decomposition
[2]. For this reason, the treatment of psoriasis with calcipotriol and betamethasone dipropionate was initially carried out by applying them separately twice a day or sequentially
[4]. The development of a formulation type of fixed dose combinations created the possibility of the simultaneous application of calcipotriol and betamethasone dipropionate, increasing their effectiveness and convenience of use, as well as patients’ compliance
[11]. The treatment is safe, systemic exposure after topical administration of calcipotriol and betamethasone dipropionate is low, and the absorption of the substances after application to healthy skin does not exceed 1%. In patients with psoriasis, the blood levels of the drugs were below the quantification level after 4 to 8 weeks of treatment
[1].
2. Supersaturated Foam Formulation of Calcipotriol/Betamethasone
Dermal and transdermal drug delivery is a continuous challenge for pharmaceutical technology. A number of various strategies of drug delivery across the skin barrier have been tested through the decades. A rich set of methods has also been proposed and tested for psoriasis treatment, e.g., laser-assisted drug delivery, foam formulations, nanoparticles, ethosomes, niomes
[12]. Among these methods, the application of supersaturated solutions is widely accepted for the treatment with calcipotriol/betamethasone formulations
[13]. The penetration of the skin by active substances after topical application is directly proportional to their concentration. Low solubility in the vehicle is a limitation for the majority of active substances in topical preparations. The chemical potential of a substance may be “artificially” increased above its solubility by using supersaturated solutions, which gives the opportunity to improve its delivery. Supersaturated solutions are thermodynamically unstable but they may be temporarily stabilized during treatment.
Supersaturation, involving the increased concentration of a substance above its vehicle solubility threshold, was introduced for foams containing calcipotriol and betamethasone
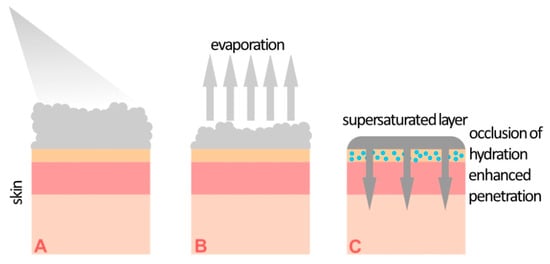
[13][14]. A supersaturated solution is formed on the skin surface ex tempore after the application of the preparation through immediate propellant evaporation. According to the information in the Summary of Product Characteristics (SmPC) of commercial foam formulations containing calcipotriol/betamethasone, the following main excipients are present: white petroleum, polyoxypropylene stearyl ether, liquid paraffin, butane, and dimethyl ether. According to their role in foam formulations, these substances may be divided into two groups, i.e., lipid anhydrous bases for calcipotriol/betamethasone and volatile solvents, which also act as propellants. The non-aqueous environment protects the active substances from decomposition due to their pH sensitivity. The processes occurring after foam application are shown in
Figure 1. After application, butane and dimethyl ether, whose boiling points are below 0 °C, quickly evaporate, leaving a supersaturated solution of calcipotriol/betamethasone in a lipid basis on the surface of the skin. A study presented by Lind et al.
[13] showed that the propellant concentration within the foam was reduced to below 2% within 30 s. Microscopy, Raman imaging, and X-ray powder diffraction (XRPD) studies confirmed that the active substances do not recrystallize in foam formulations for at least 18 h, and probably much longer. In contrast, crystals were observed immediately after the application of a standard ointment formulation. Research on the penetration of calcipotriol and betamethasone dipropionate through pig ear skin confirmed a statistically significant increase in active substance concentrations in comparison with the concentrations reached when using an ointment. From a practical point of view, the occlusive properties of the supersaturated layer are very important. They increase the hydration of the stratum corneum by inhibiting water evaporation, which improves skin permeability.
Figure 1. Illustration of the formation of a supersaturated layer on the skin after the administration of a calcipotriol/betamethasone foam. (A) Foam application, (B) solvent evaporation, (C) formation of a supersaturated layer.
Both in vitro and in vivo research showed the superiority in active substances’ speed of penetration and concentration reached when using foams compared to gels or ointments
[13].
The outcomes obtained with the use of foams support the view of an increasing number of dermatologists that foams leading to supersaturation of calcipotriol and betamethasone will change dermatology and clinical practice as regards the treatment of psoriasis
[15][16].
3. Comparison of Foam and Ointment
A double-blind multicenter phase II study
[17] compared the effectiveness and safety of two preparations containing calcipotriol and betamethasone dipropionate—a foam and an ointment. The study included a total of 376 patients. The primary endpoint to evaluate the formulations’ effectiveness was the percentage of patients whose skin lesions regressed or almost completely regressed as confirmed by Physicians Global Assessment (PGA) analysis after 4 weeks. The number of patients in whom therapeutic success was achieved was significantly higher in the group who had used the foam formulation compared to the group who had used the ointment (54.6% and 43.0%, respectively,
p = 0.025). Moreover, assessment with the mPASI method (modification of the Psoriasis Area and Severity Index, which excludes the hairy scalp on which no foam/ointment was applied) demonstrated a statistically significant advantage of foam over ointment at both assessed time points (1 week and 4 weeks). The authors concluded that the effectiveness of the foam formulation was markedly higher than that of the ointment, with a comparable safety profile.
The standard vasoconstriction test for the evaluation of glucocorticoid effects was used to compare the activities of a foam containing calcipotriol and betamethasone and an ointment containing betamethasone (no calcipotriol). The degree of vasoconstriction obtained with the foam was (median) 2.00 points, while that achieved with the ointment containing betamethasone but without calcipotriol was 1.75 points, with the difference being statistically insignificant (
p = 0.30)
[18].
An analysis of the cost effectiveness of foams and ointments containing calcipotriol and betamethasone was conducted in Sweden
[19]. A relatively complex organizational regimen involved the application of a foam or an ointment prior to systemic treatment. A significantly higher effectiveness of the foam was observed in comparison with the ointment. The use of foam was associated with a lower number of medical consultations and a lower percentage of patients for whom systemic treatment was necessary.
4. Comparison of Foam and Gel
A 12-week PSO-ABLE phase III study
[20] was conducted to compare the therapeutic effectiveness and safety of a foam containing calcipotriol and betamethasone and a gel containing the same amount of active substances. The study included 463 patients. The average baseline BSA was 7.1 ± 5.7 in the group of patients using the foam and 7.0 ± 5.5 in the group who used the gel. The primary endpoint of effectiveness assessment was the percentage of patients in whom therapeutic success was achieved. Therapeutic success was defined according to the PGA scale (0–4) as “no lesions” in case of patients with mild lesions at baseline and “no lesions” or “almost no lesions” in patients with moderate or severe psoriasis at baseline. On the basis of the above definition, the effectiveness of the foam was characterized as markedly higher compared to that of the gel. The percentages of patients in whom therapeutic success was achieved (“no” or “almost no” psoriatic lesions) were 38.3% and 19.6% after 4 weeks for the foam and gel groups, respectively, while after 8 weeks, the respective percentages were 44.5% and 22.5%. The study also showed that, after 4 weeks, therapeutic success was achieved in a significantly higher percentage of patients using the foam, whereas it required 8 weeks in patients using the gel. A similar difference was observed when analyzing the effectiveness with mPASI. During the study, the patients used on average 98.6 g of foam and 164.3 g of gel (after 4 and 8 weeks, respectively). They used 236.4 g of foam and 193.1 g of gel over 12 weeks.
The secondary endpoints of effectiveness were the percentage of patients achieving at least 75% of modified PASI (mPASI75) reduction and the time to treatment success. A significant advantage of the foam was demonstrated also for those parameters. The authors emphasized that the median time for achieving the standard index of improvement of mPASI75 was 4 weeks for the foam and 12 weeks for the gel.
A phase III clinical trial also assessed the influence of using foam and gel formulations on patients’ quality of life
[21]. The following scales were used in the assessment: Health-Related Quality of Life (HRQoL), including the Dermatology Life Quality Index (DLQI), the EuroQoL-5D-5L-PSO (EQ-5D), and the Psoriasis QoL (PQoL-12). Moreover, the researchers evaluated such variables as pruritus, sleep deprivation triggered by pruritus, and the influence of the disease on the working life. The study included 463 patients with plaque psoriasis with BSA of 2 to 30%. In this group, 185 patients applied a foam, 188 used a gel formulation, 47 used a foam vehicle without active substances, and 43 used a gel vehicle. DLQI 0 or 1 were obtained by considerably more patients using the foam rather than the gel at week 4 (45.7% vs 32.4%, respectively;
p = 0.013) and at week 12 (60.5% vs 44.1%, respectively;
p = 0.003). The foam was also more effective as regards other parameters concerning the quality of life, including EQ-5D (0.09 vs 0.03;
p < 0.001) and PQoL-12 (−2.23 vs −2.07;
p = 0.029), and in terms of the influence on pruritus, pruritus-related sleep deprivation, and work impairment.
Another phase III clinical trial
[22] evaluated the preferences of patients concerning the vehicle. The authors compared a foam containing calcipotriol with a gel containing the same active substance. “The previous treatment” was the reference point. It was a prospective multicenter study (NCT02310646). The foam was used once daily for 7 days and then was substituted by the gel or the opposite. The study included 213 patients. For some parameters, the patients claimed they preferred the foam, e.g., because of immediate relief, the soothing quality of the preparation, or the feeling of alleviating the condition. As regards the gel, the patients indicated its easy application or easy spreading.