
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | FUXIN LIAN | + 2421 word(s) | 2421 | 2021-05-11 04:54:27 | | | |
| 2 | Dean Liu | -10 word(s) | 2411 | 2021-12-10 01:43:48 | | |
Video Upload Options
Autism spectrum disorder (ASD) is characterized by a fundamental change in self-awareness including seemingly paradoxical features like increased ego-centeredness and weakened self-referentiality.
1. Introduction
Autism spectrum disorder (ASD) is a complex psychiatric condition that is characterized by multiple symptoms. Cognitive symptoms like changes in autobiographical/episodic memory [1][2][3][4] are coupled with deficits in social cognition as in theory of mind [5][6][7], affective changes including emotion, empathy, and facial expression [8][9][10], hypersystemizing [11], motor symptoms like difficulty of action imitation [12][13][14], stereotypies and repetitions [15][16][17], and multimodal sensory integration [18][19][20][21][22]. Yet, on a deeper level beneath the various functions, an altered sense of self, i.e., self-awareness, has been described and is a key disturbance of autism [23].
In their original descriptions of autism, Kanner (1943) and Asperger (1944) point out a fundamental or basic disturbance of self: the self in ASD is only himself and self-sufficient [24] and feels neither an integral part of the world nor stands in a lively dynamic relationship with its environment [25]. More recently, Lombardo and Baron-Cohen (2010, 2011) highlighted what they describe as “paradox of self” in ASD [23][26] (see also [27][28][29]). On the one hand, the autistic self is highly centered on itself, showing an abnormally high degree of ego-centeredness as manifest in social isolation and loneliness, inability to read the emotions, feelings, and facial expressions of others [30][31][32], and major deficits in social cognition like theory of mind [5][6][7]. Such high ego-centeredness is, on the other hand, contrasted by weak self-referentiality with decreased use of “I” in language [33][34][35], no mention of own internal states, e.g., own emotion [10][36][37], own theory of mind [27][38][39], changes in time processing like duration estimation of shorter and longer time intervals as deficits in connecting different time points [40][41][42], decreased introspection [43][44], decrease in interoception [45][46][47], and reduced autobiographical memory [1][2][3][4] (see though Markram and Markram, 2012 [48], as well as Lind et al., 2020 [49]).
How is it possible that seemingly two contradictory features like increased ego-centeredness and decreased self-referentiality can co-occur within one and the same person’s self? Lombardo and Baron-Cohen (2010) assume a shared deficit in the neural circuitry that encodes self-representation, including self–other distinction and self–other awareness [26]. Various imaging studies, most often using fMRI, investigated self-reference in ASD. They observed changes in various anterior and posterior regions of the default-mode network (DMN) and outside the DMN during self-referential tasks (see below). At the same time, resting state abnormalities could also be observed in anterior and posterior DMN (and also non-DMN) of ASD, which again showed major changes in DMN (see for recent review Lau et al., 2020 [50] and below). Given that various findings in healthy subjects indicate neural overlap of self and DMN resting state [51][52][53][54][55][56], one may assume a close relationship of resting state and self-specific task-related changes in DMN of ASD. The goal of the present paper is to review recent fMRI findings in ASD during both rest and self-referential task states in order to reconcile the seemingly features of increased ego-centeredness and decreased self-referentiality.
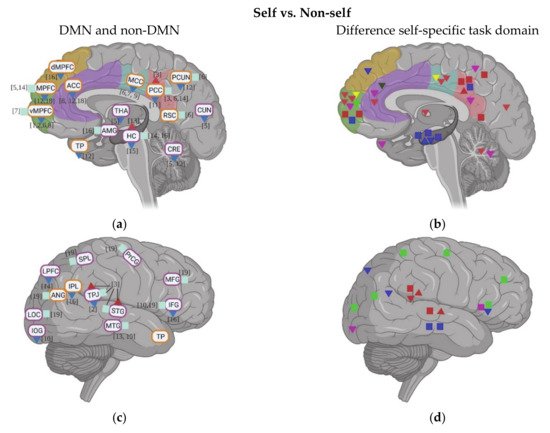
A recent meta-analytic study on the self in healthy subjects suggests a multilayered nested hierarchical model of self with interoceptive self, exteroceptive self, and mental self: neural correlates range from subcortical regions and insula (interoceptive self) over medial prefrontal cortex and temporo-parietal junction (TPJ) (exteroceptive self) to anterior and posterior DMN (mental self) [57][58].
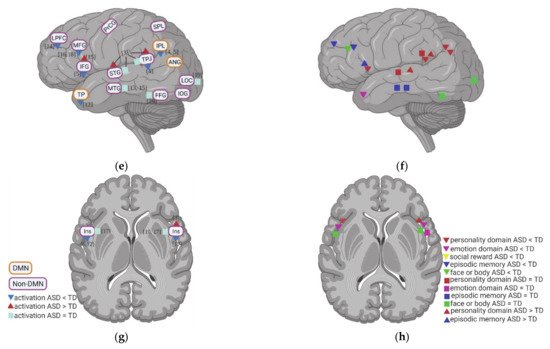
2. Task fMRI in ASD: Task-Related Neural Activity and Functional Connectivity during Self
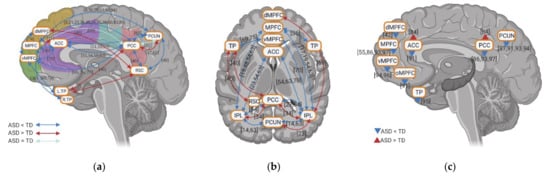
3. Resting State fMRI in ASD: Resting State Functional Connectivity (rsFC) within DMN
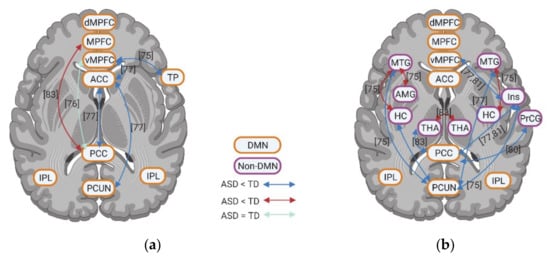
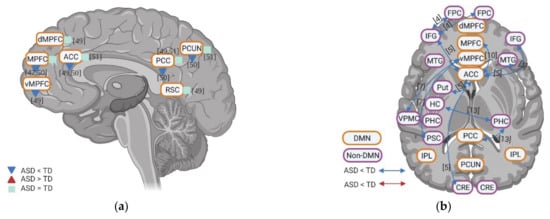
4. Resting State fMRI in ASD: Resting State Functional Connectivity between DMN and Non-DMN
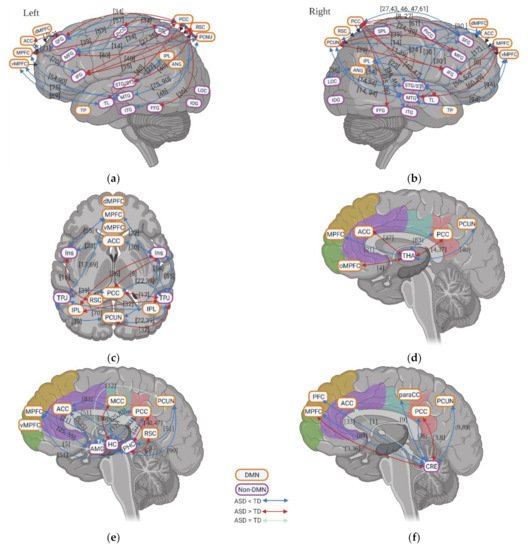
5. Resting State fMRI in ASD: Subcortical–Cortical Resting State Functional Connectivity
6. Resting State fMRI in ASD: ALFF and REHO in DMN Regions
Few studies investigated the intraregional resting state activity using amplitude of low-frequency fluctuations (ALFF) and regional homogeneity (ReHo), in individuals with ASD compared to TD controls. In these studies, relatively consistent results of the intraregional activity of the DMN were reported. Anterior DMN regions (e.g., MPFC, ACC, and TP) have reduced activity in ReHo. Meanwhile, although two studies reported hyperactivity in PCUN in ReHo, the posterior DMN regions (e.g., PCC and PCUN) show decreased activity in both ALFF and ReHo in ASD (Figure 6). In general, findings show decreased intraregional resting state activity in anterior and posterior DMN regions in ASD.
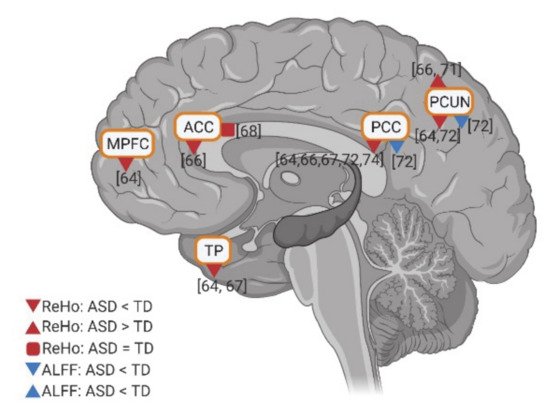
Figure 6. Intraregional resting state activity (ALFF and REHO) in DMN for individuals with ASD compared to TD. The numbers in the figure correspond to the study numbers in Table 2.
References
- Terrett, G.; Rendell, P.G.; Raponi-Saunders, S.; Henry, J.D.; Bailey, P.E.; Altgassen, M. Episodic Future Thinking in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2013, 43, 2558–2568.
- Lind, S.E.; Williams, D.M.; Bowler, D.M.; Peel, A. Episodic memory and episodic future thinking impairments in high-functioning autism spectrum disorder: An underlying difficulty with scene construction or self-projection? Neuropsychology 2014, 28, 55–67.
- Cygan, H.B.; Marchewka, A.; Kotlewska, I.; Nowicka, A. Neural Correlates of Reflection on Present and Past Selves in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 1267–1277.
- Hogeveen, J.; Krug, M.K.; Geddert, R.M.; Ragland, J.D.; Solomon, M. Compensatory Hippocampal Recruitment Supports Preserved Episodic Memory in Autism Spectrum Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 97–109.
- Senju, A.; Southgate, V.; White, S.; Frith, U. Mindblind eyes: An absence of spontaneous theory mind in Asperger Syndrome. Science 2009, 325, 883–885.
- Baron-Cohen, S.; Leslie, A.M.; Frith, U. Does the autistic child have a “theory of mind”? Cognition 1985, 21, 37.
- Firth, U. Mind Blindness and the Brain in Autism. Neuron 2001, 32, 969–979.
- Harmsen, I.E. Empathy in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 3939–3955.
- Tanaka, J.W.; Sung, A. The "Eye Avoidance" Hypothesis of Autism Face Processing. J. Autism Dev. Disord. 2016, 46, 1538–1552.
- Kinnaird, E.; Stewart, C.; Tchanturia, K. Investigating alexithymia in autism: A systematic review and meta-analysis. Eur. Psychiat. 2019, 55, 80–89.
- Baron-Cohen, S.; Lombardo, M.V. Autism and talent: The cognitive and neural basis of systemizing. Dialogues Clin. Neurosci. 2017, 19, 345–353.
- Bernier, R.; Dawson, G.; Webb, S.; Murias, M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain Cogn. 2007, 64, 228–237.
- Dewey, D.; Cantell, M.; Crawford, S.G. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. 2007, 13, 246–256.
- Chetcuti, L.; Hudry, K.; Grant, M.; Vivanti, G. Object-directed imitation in autism spectrum disorder is differentially influenced by motoric task complexity, but not social contextual cues. Autism 2019, 23, 199–211.
- Leekam, S.R.; Prior, M.R.; Uljarevic, M. Restricted and repetitive behaviors in autism spectrum disorders: A review of research in the last decade. Psychol. Bull. 2011, 137, 562–593.
- Matson, J.L.; Dempsey, T.; Fodstad, J.C. Stereotypies and repetitive/restrictive behaviours in infants with autism and pervasive developmental disorder. Dev. Neurorehabil. 2009, 12, 122–127.
- Watt, N.; Wetherby, A.M.; Barber, A.; Morgan, L. Repetitive and Stereotyped Behaviors in Children with Autism Spectrum Disorders in the Second Year of Life. J. Autism Dev. Disord. 2008, 38, 1518–1533.
- Simon, D.M.; Wallace, M.T. Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016, 68, 848–861.
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160.
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci 2017, 18, 671–684.
- Stevenson, R.A.; Siemann, J.K.; Woynaroski, T.G.; Schneider, B.C.; Eberly, H.E.; Camarata, S.M.; Wallace, M.T. Evidence for Diminished Multisensory Integration in Autism Spectrum Disorders. J. Autism Dev. Disord. 2014, 44, 3161–3167.
- Siemann, J.K.; Veenstra-VanderWeele, J.; Wallace, M.T. Approaches to Understanding Multisensory Dysfunction in Autism Spectrum Disorder. Autism Res. 2020, 13, 1430–1449.
- Lombardo, M.V.; Baron-Cohen, S. The role of the self in mindblindness in autism. Conscious. Cogn. 2011, 20, 130–140.
- Kanner, L. Autistic disturbances of affective contact. Nervous Child. 1943, 2, 217–250.
- Asperger, H. Die “Autistischen Psychopathen” im Kindesalter. Archivf. Psychiatr. 1944, 117, 76–136.
- Lombardo, M.V.; Baron-Cohen, S. Unraveling the paradox of the autistic self. Wires Cogn. Sci. 2010, 1, 393–403.
- Williams, D. Theory of own mind in autism: Evidence of a specific deficit in self-awareness? Autism 2010, 14, 474–494.
- Uddin, L.Q. The self in autism: An emerging view from neuroimaging. Neurocase 2011, 17, 201–208.
- Perrykkad, K.; Hohwy, J. Modelling Me, Modelling You: The Autistic Self. Rev. J. Autism Dev. Dis. 2020, 7, 1–31.
- Uljarevic, M.; Hamilton, A. Recognition of Emotions in Autism: A Formal Meta-Analysis. J. Autism Dev. Disord. 2013, 43, 1517–1526.
- Harms, M.B.; Martin, A.; Wallace, G.L. Facial Emotion Recognition in Autism Spectrum Disorders: A Review of Behavioral and Neuroimaging Studies. Neuropsychol. Rev. 2010, 20, 290–322.
- Dapretto, M.; Davies, M.S.; Pfeifer, J.H.; Scott, A.A.; Sigman, M.; Bookheimer, S.Y.; Iacoboni, M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006, 9, 28–30.
- Finnegan, E.G.; Asaro-Saddler, K.; Zajic, M.C. Production and comprehension of pronouns in individuals with autism: A meta-analysis and systematic review. Autism 2020, 230534210.
- Naigles, L.R.; Cheng, M.; Xu Rattanasone, N.; Tek, S.; Khetrapal, N.; Fein, D.; Demuth, K. “You’re telling me!” The prevalence and predictors of pronoun reversals in children with autism spectrum disorders and typical development. Res. Autism Spect. Dis. 2016, 27, 11–20.
- Hobson, R.P.; Lee, A.; Hobson, J.A. Personal Pronouns and Communicative Engagement in Autism. J. Autism Dev. Disord. 2010, 40, 653–664.
- Silani, G.; Bird, G.; Brindley, R.; Singer, T.; Frith, C.; Frith, U. Levels of emotional awareness and autism: An fMRI study. Soc. Neurosci. UK 2008, 3, 97–112.
- Hill, E.; Berthoz, S.; Frith, U. Brief Report: Cognitive Processing of Own Emotions in Individuals with Autistic Spectrum Disorder and in Their Relatives. J. Autism Dev. Disord. 2004, 34, 229–235.
- Williams, D.; Happe, F. Representing intentions in self and other: Studies of autism and typical development. Dev. Sci 2010, 13, 307–319.
- Williams, D.M.; Happe, F. What did I say? Versus what did I think? Attributing false beliefs to self amongst children with and without autism. J. Autism Dev. Disord. 2009, 39, 865–873.
- Casassus, M.; Poliakoff, E.; Gowen, E.; Poole, D.; Jones, L.A. Time perception and autistic spectrum condition: A systematic review. Autism Res. 2019, 12, 1440–1462.
- Jurek, L.; Longuet, Y.; Baltazar, M.; Amestoy, A.; Schmitt, V.; Desmurget, M.; Geoffray, M. How did I get so late so soon? A review of time processing and management in autism. Behav. Brain Res. 2019, 374, 112121.
- Isaksson, S.; Salomäki, S.; Tuominen, J.; Arstila, V.; Falter-Wagner, C.M.; Noreika, V. Is there a generalized timing impairment in Autism Spectrum Disorders across time scales and paradigms? J. Psychiatr. Res. 2018, 99, 111–121.
- Robinson, S.; Howlin, P.; Russell, A. Personality traits, autobiographical memory and knowledge of self and others: A comparative study in young people with autism spectrum disorder. Autism 2017, 21, 357–367.
- Greimel, E.; Schulte-Rüther, M.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Konrad, K. Selbst- und Fremdbeurteilung der Empathie bei Jugendlichen mit Autismus. Z. Kinder Jugendpsychiatrie Psychother. 2011, 39, 113–121.
- Nicholson, T.; Williams, D.; Carpenter, K.; Kallitsounaki, A. Interoception is Impaired in Children, But Not Adults, with Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 3625–3637.
- Nicholson, T.M.; Williams, D.M.; Grainger, C.; Christensen, J.F.; Calvo-Merino, B.; Gaigg, S.B. Interoceptive impairments do not lie at the heart of autism or alexithymia. J. Abnorm. Psychol. 2018, 127, 612–622.
- Palser, E.R.; Fotopoulou, A.; Pellicano, E.; Kilner, J.M. The link between interoceptive processing and anxiety in children diagnosed with autism spectrum disorder: Extending adult findings into a developmental sample. Biol. Psychol. 2018, 136, 13–21.
- Markram, K.; Markram, H. The intense world theory—a unifying theory of the neurobiology of autism. Front. Hum. Neurosci. 2010, 4, 224.
- Lind, S.E.; Williams, D.M.; Nicholson, T.; Grainger, C.; Carruthers, P. The self-reference effect on memory is not diminished in autism: Three studies of incidental and explicit self-referential recognition memory in autistic and neurotypical adults and adolescents. J. Abnorm. Psychol. 2020, 129, 224–236.
- Lau, W.K.W.; Leung, M.; Zhang, R. Hypofunctional connectivity between the posterior cingulate cortex and ventromedial prefrontal cortex in autism: Evidence from coordinate-based imaging meta-analysis. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 103, 109986.
- D’Argembeau, A.; Collette, F.; Van der Linden, M.; Laureys, S.; Del, F.G.; Degueldre, C.; Luxen, A.; Salmon, E. Self-referential reflective activity and its relationship with rest: A PET study. Neuroimage 2005, 25, 616–624.
- Schneider, F.; Bermpohl, F.; Heinzel, A.; Rotte, M.; Walter, M.; Tempelmann, C.; Wiebking, C.; Dobrowolny, H.; Heinze, H.J.; Northoff, G. The resting brain and our self: Self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 2008, 157, 120–131.
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? Neuroimage 2011, 57, 1221–1233.
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. Neuroimage 2016, 132, 390–397.
- Kolvoort, I.R.; Wainio-Theberge, S.; Wolff, A.; Northoff, G. Temporal integration as “common currency” of brain and self-scale-free activity in resting-state EEG correlates with temporal delay effects on self-relatedness. Hum. Brain Mapp. 2020, 41, 4355–4374.
- Wolff, A.; Di Giovanni, D.A.; Gomez-Pilar, J.; Nakao, T.; Huang, Z.; Longtin, A.; Northoff, G. The temporal signature of self: Temporal measures of resting-state EEG predict self-consciousness. Hum. Brain Mapp. 2019, 40, 789–803.
- Qin, P.; Grimm, S.; Duncan, N.W.; Fan, Y.; Huang, Z.; Lane, T.; Weng, X.; Bajbouj, M.; Northoff, G. Spontaneous activity in default-mode network predicts ascription of self-relatedness to stimuli. Soc. Cogn. Affect. Neurosci. 2016, 11, 693–702.
- Qin, P.; Wang, M.; Northoff, G. Linking bodily, environmental and mental states in the self—A three-level model based on a meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 77–95.




