Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jing Han | + 2399 word(s) | 2399 | 2021-12-08 07:40:22 | | | |
| 2 | Yvaine Wei | Meta information modification | 2399 | 2021-12-09 02:48:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Han, J. 4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/16875 (accessed on 07 February 2026).
Han J. 4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/16875. Accessed February 07, 2026.
Han, Jing. "4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications" Encyclopedia, https://encyclopedia.pub/entry/16875 (accessed February 07, 2026).
Han, J. (2021, December 08). 4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications. In Encyclopedia. https://encyclopedia.pub/entry/16875
Han, Jing. "4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications." Encyclopedia. Web. 08 December, 2021.
Copy Citation
Polyhydroxyalkanoates (PHA) are polyesters having high promise in biomedical applications. Among different types of PHA, poly-4-hydroxybutyrate (P4HB) is the only polymer that has received FDA approval for medical applications. However, most PHA producing microorganisms lack the ability to synthesize P4HB or PHA comprising 4-hydroxybutyrate (4HB) monomer due to their absence of a 4HB monomer supplying pathway. Thus, most microorganisms require supplementation of 4HB precursors to synthesize 4HB polymers.
microbial synthesis
polyhydroxyalkanoates
4HB-containing PHA
1. Introduction
As the global concerns about environmental sustainability is rising, bioplastics are gaining increasing importance as a viable alternative to petrochemical-based plastics [1]. Bioplastics are environmentally friendly, biodegradable materials derived from renewable sources. Polyhydroxyalkanoates (PHA) are polyesters usually produced by various bacteria and archaea as carbon and energy storage under unbalanced growth conditions of limiting nutrients and excess carbon supply [2]. Besides complete biodegradability in nature, the mechanical, crystal, and thermal properties of PHA are almost comparable with the petroleum counterparts [3]. Importantly, properties of PHA polymers somehow depend on their monomer composition. By changing the monomer type and composition, PHA with favorable material properties can be achieved [4]. There are at least 150 different hydroxyalkanoates that have been identified as PHA monomers [5]. Depending upon the carbon length of these monomers, PHA are classified as short-chain length (SCL-PHA, C3-C5 monomer), medium-chain length (MCL-PHA, C6-C14 monomer), and long-chain length (LCL-PHA, more than 14 carbons in monomer) [6].
SCL-PHA are the most commonly synthesized polymers by bacteria and archaea. Three monomers of SCL-PHA that have received considerable attention are 3-hydroxybutyrate (3HB), 3-hydroxyvalerate (3HV), and 4-hydroxybutyrate (4HB). Polyhydroxybutyrate (PHB), the homopolymer of 3HB, is the most widely produced PHA. PHB is brittle and highly crystalline in nature [7]. It has a high melting point and narrow processing window that makes its industrial processing difficult [8]. Interestingly, the copolymer of 3HB and 3HV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) shows enhanced mechanical properties like tensile strength, toughness, and Young’s modulus, and also reduced melting point [9]. The polymer becomes less crystalline that improves its processing ability. Notably, the homopolymer of 4HB, poly-4-hydroxybutyrate (P4HB), features superior properties and it is the only PHA that has received FDA approval for medical applications since 2007 [10].
2. Medical Application of P4HB
P4HB is a strong, pliable, and resorbable thermoplastic biomaterial [11]. P4HB is extremely elastic, and its tensile properties are almost comparable to the ultra-high-molecular-weight polyethylene [12]. It is highly ductile in nature and has an elongation to break of 1000%, indicating that it can be stretched ten times to its original size before breaking [13]. P4HB has excellent thermal processability and can be fairly molded into various structures including fibers, films, tubes, and microspheres [10]. Tepha’s TephaFLEX® is a P4HB biopolymer. Interestingly, the monofilament sutures fabricated using this P4HB polymer was 35% stronger than the synthetic polydioxanone suture and 19% stronger than the polypropylene suture [14]. Therefore, P4HB fibers have been used as the starting material for medical textile products like patches, grafts, surgical meshes, etc. [15]. Phasix™ mesh is a resorbable biosynthetic mesh prepared from P4HB [16]. Interestingly, it provided long-term mechanical strength during hernia repair surgery [17]. This might prevent further surgical complications and reduce the rate of hernia recurrence. It has also been observed that the P4HB-based surgical mesh reduced the chances of post-operative surgical site infections [18]. Thus, Phasix™ may become a treatment option for hernia repair. Furthermore, P4HB are being successfully implemented in tissue engineering. Compared to polyglycolic acid (PGA) mesh, P4HB scaffolds showed prolonged degradation and also promoted tissue regeneration [19]. P4HB-based scaffold for trileaflet heart valves, vascular grafts, and artery augmentation, and P4HB pelvic floor implants are already under consideration [14][20]. Moreover, degradation of P4HB does not produce acidic by-products at wound sites and elicits mild inflammatory response during wound healing [21]. Due to its unique characteristics, P4HB is recognized as a promising biodegradable and biocompatible biopolymer having immense potentials in biomedical engineering. However, high production cost is still an obvious challenge faced by PHA research. To commercialize the use of P4HB and make it available for use in daily lives, it is important to curb down the production cost of P4HB.
3. Microbial Synthesis of P4HB
Several methods have been adopted to synthesize P4HB chemically. However, due to the involvement of various organometallic or metal complex catalysts such as Lewis acid, lanthanum amide, distannoxane complex, and titanium, chemical synthesis of P4HB is not suitable for biomedical applications [10]. Thus, P4HB synthesis through fermentation processes is the most desirable approach. Unlike PHB, a major drawback of microbial P4HB production is that the naturally occurring P4HB-producing microbes are very limited. Most microbes can’t produce the P4HB homopolymer rather they tend to incorporate 3HB and/or 3HV monomers, resulting in the formation of 4HB copolymers or terpolymers. Both 4HB co- and terpolymers have novel and fascinating properties that are favoured over PHB and PHBV. Therefore, incorporating different proportions of 3HB, 3HV, and 4HB monomers to yield tailor-made co- or terpolymer with desirable characteristics is an interesting research area. However, the fine balance between the monomeric units is critical. Sometimes, a high 3HB and 3HV content overshadows the benefits of 4HB content [22]. Therefore, optimizing the monomer composition is often challenging and requires extensive investigation. Taken together, a high 4HB content is always preferable and thus, continuous efforts are being made to synthesize PHA polymers having a high 4HB molar fraction. In addition, obtaining 4HB polymers at reduced cost is also a challenge faced by researchers in this field.
3.1. Natural 4HB Polymer Producers and 4HB-CoA Supplying Pathway
Hydrogenophaga pseudoflava is one of the few known microbes capable of producing 4HB polymer from structurally unrelated carbon sources. Using L-arabinose as the substrate, the strain synthesized the terpolymer P(3HB-co-1 mol% 3HV-co-5 mol% 4HB) with a PHA content of 45.3 wt% (CDW) [23]. Thus, H. pseudoflava is a potentially important strain that should be further investigated to understand the metabolic pathway involved in 4HB polymer synthesis from unrelated carbon sources.
Synthesis of 4HB polymer requires generation of the 4HB-CoA monomer. Using structurally unrelated carbon sources like glucose, 4HB-CoA is synthesized from succinyl-CoA [13]. The synthesis of 4HB monomer from the succinate degradation pathway was characterized in Clostridium kluyveri [24]. In this pathway, succinate is converted to succinyl-CoA by succinyl-CoA: CoA transferase. The latter is then converted into succinate semialdehyde (SSA) by SSA dehydrogenase (Figure 1). The SSA is reduced to 4HB by 4HB dehydrogenase. 4HB-CoA: CoA transferase converts 4HB to 4HB-CoA. However, this strain is not able to synthesize 4HB polymer rather 4-hydroxybutyryl-CoA dehydratase/vinylacetyl-CoA Δ3-Δ2-isomerase converts 4HB-CoA to crotonyl-CoA. For synthesis of 4HB polymer, under the action of PHA synthase, 4HB-CoA is incorporated into the polymer chain instead of channelizing it to other pathways. Most often, the genes involved in the 4HB-CoA generation pathway is absent or suppressed in microbes and thus, they are incapable of synthesizing 4HB polymer from unrelated carbon sources. This necessitates supplementation of structurally related 4HB precursors for the incorporation of 4HB monomers into polymer chain.
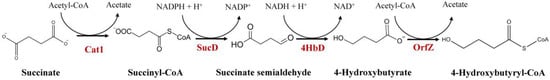
Figure 1. Naturally occurring 4HB-CoA supplying pathway in Clostridium kluyveri. Cat1, succinyl-CoA:CoA transferase; SucD, succinate semialdehyde dehydrogenase; 4HbD, 4-hydroxybutyrate dehydrogenase; OrfZ, 4HB-CoA: CoA transferase.
3.2. Precursor Supplementation to Yield 4HB Copolymers
Precursor supplementation is the most important prerequisite for synthesis of 4HB polymers in many microbes. The commonly used 4HB precursors include γ-butyrolactone, 1,4-butanediol, and 1,6-hexanediol. These precursors are metabolized to form 4HB, and then 4HB is converted to 4HB-CoA by 4HB-CoA: CoA transferase.
Studies on 4HB polymers dates back to late 1980s. For the first time, the synthesis of 4HB-containing PHA polymer was observed in Cupriavidus necator (also known as Ralstonia eutropha or Alcaligenes eutrophus) [25]. The strain produced PHB in the presence of carbon sources like fructose or butyric acid. However, co-feeding of γ-butyrolactone resulted in the synthesis of P(3HB-co-4HB) copolymer. γ-butyrolactone alone increased the 4HB content to 21 mol% in the polymer. Co-feeding γ-butyrolactone with fructose decreased the 4HB content to 4 mol% as fructose led to the formation of more 3HB-CoA moieties. Strikingly, the mixture of γ-butyrolactone and butyric acid yielded a higher 4HB molar fraction (24 mol%), indicating that butyric acid also contributed to the formation of 4HB-CoA monomer [25]. As the 4HB molar fraction increased in the copolymer, its crystallinity decreased and elongation to break increased. At 3 mol% of 4HB, crystallinity of the copolymer was 55% and elongation to break was 45%. Interestingly at 16 mol%, the crystallinity decreased to 45% and the elongation to break increased to 444%. Finally, due to the parameters’ change, the physical properties of the polymer improved [25]. P(3HB-co-4HB) copolymer with a high 4HB molar fraction is more preferable for biomedical applications.
3.3. Microbial Synthesis of 4HB Terpolymer
Studies on the synthesis of 4HB terpolymer is considerably limited. Till now, few microbes namely C. necator, C. malaysiensis, Aneurinibacillus sp. H1, H. pseudoflava, and haloarchaeon Haloferax mediterranei have been reported to synthesize terpolymer P(3HB-co-3HV-co-4HB). Using 4-hydroxybutyric acid or γ-butyrolactone as 4HB precursor and propionic acid as 3HV precursor along with glucose, C. necator produced several types of the terpolymer P(3HB-co-3HV-co-4HB) [26]. However, the terpolymer comprising approximately 10 mol% of 3HV and 4HB each showed better mechanical properties than P(3HB-co-30 mol% 3HV). Changing the carbon source type and ratio further changed the 4HB molar fraction in its terpolymer by using C. necator. For example, the supplementation of 4-hydroxybutyric acid to the medium containing fructose or butyric acid, and valerate resulted in the production of terpolymer containing 4HB up to 84 mol% [27]. Moreover, the 4HB content further increased to 93 mol% with increasing cultivation time. Replacing the 4HB precursor with 1,4-butanediol, however, reduced the 4HB content to almost 60 mol% in this strain. A thermophilic gram-positive bacterium Aneurinibacillus sp. H1 accumulated PHB when cultured on glucose or glycerol. However, when supplemented with 1,4-butanediol, the strain produced P(3HB-co-4HB) with 4HB molar fraction up to 90 mol%. Moreover, by cofeeding valerate, it was able to synthesize terpolymer P(3HB-co-33 mol% 3HV-co-54 mol% 4HB) [28].
4. Metabolic Engineering to Synthesize 4HB Polymers
Supplementation of 4HB precursor for synthesis of 4HB-containing polymer incurs additional production cost. Moreover, some microbes including E. coli and Halomonas bluephagenesis TD01 are even incapable of utilizing 4HB precursors as carbon source. Thus, metabolic engineering is a better solution to enable 4HB polymer synthesis in such microbes. Pathway engineering that includes heterologous 4HB synthesis pathway de novo construction has successfully allowed 4HB polymer synthesis in several microbes.
4.1. Construction of Recombinant E. coli to Synthesize 4HB Polymer
Wild type E. coli is incapable of synthesizing PHA due to the lack of pha synthesis genes. However, PHB synthesis in E. coli has been made possible by pathway engineering. Similarly, recombinant E. coli was able to synthesize 4HB polymer when 4HB synthesis pathway was established in this strain (Figure 2). The entire PHA synthase gene (phaC) and 878 of 1179-bp of the 5′ region of the β-ketothiolase gene (phaA) from C. necator plus the entire 4HB-CoA transferase gene (orfZ) from Clostridium kluyveri were heterologously expressed in E. coli [29]. Supplementing M9 mineral salt medium with 4-hydroxybutyric acid and glucose, the recombinant E. coli synthesized ~80 wt% (CDW) P4HB homopolymer under oxygen-deficient condition. Even when replacing 4-hydroxybutyric acid with γ-butyrolactone, the E. coli strain synthesized 16.1 wt% (CDW) P4HB homopolymer.
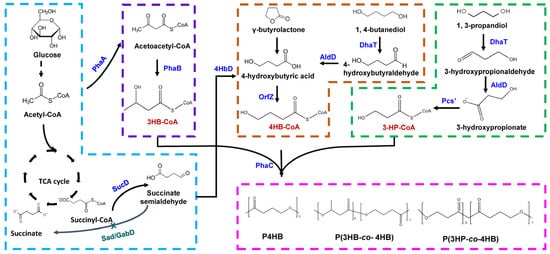
Figure 2. Different metabolic engineering strategies to synthesize 4HB-containing PHA in Escherichia coli. When using glucose as the sole substrate, it is converted to succinyl-CoA via glycolytic pathway and TCA cycle. Succinyl-CoA is converted to succinate semialdehyde by SucD. Deletion of native sad/gabD genes prevents the conversion of succinate semialdehyde to succinate. Then, succinate semialdehyde is converted to 4-hydroxybutyric acid by 4HbD. When using γ-butyrolactone or 1, 4-butanediol as 4HB precursor, they are converted to 4-hydroxybutyric acid. 4-hydroxybutyric acid is then converted to 4HB-CoA by OrfZ. 3HB-CoA is derived from acetyl-CoA under the action of PhaA and PhaB. Finally, P4HB or P(3HB-co-4HB) is synthesized under the action of PhaC. For the synthesis of P (3HP-co-4HB), 3HP-CoA is derived from 1,3-propandiol. Under the action of PhaC, 3HP-CoA and 4HB-CoA is polymerized to form P (3HP-co-4HB). SucD, succinate semialdehyde dehydrogenase; Sad and GabD, succinate semialdehyde dehydrogenase; PhaA, β-ketothiolase; PhaB, acetoacetyl-CoA reductase; PhaC, PHA synthase; 4hbD, 4-hydroxybutyrate dehydrogenase; OrfZ, CoA transferase; DhaT, 1,3-propanediol dehydrogenase; AldD, aldehyde dehydrogenase; Pcs’, ACS domain of propionyl-CoA synthase.
4.2. Engineering H. bluephagenesis TD01 for Synthesis of 4HB Polymer
H. bluephagenesis TD01 is a halophilic bacterium showing a high capability in PHA industrial production, however, it is unable to synthesize 4HB-containing PHA even in the presence of 4HB precursor. The 4HB-CoA transferase encoding gene is absent in this microbe. Hence, the orfZ gene from C. kluyveri was expressed in H. bluephagenesis TD01(Figure 3) [30]. Unlike E. coli, H. bluephagenesis TD01 possesses its own pha synthesis genes. The T7-like system was used to tune the orfZ expression strength. In the presence of glucose and γ-butyrolactone, the recombinant strain incorporated 11.2 mol% of 4HB in P(3HB-co-4HB). To ensure better genetic stability, orfZ was then chromosomally integrated in the genome of H. bluephagenesis TD01. In a 7-L fermentor, 72 g/L CDW containing 63% P(3HB-co-12.3 mol% 4HB) was produced in 48 h. To further scale-up the process, the fermentation was carried out in 1000-L fermentor in an open unsterile condition. After 48 h of fermentation, 82.6 g/L CDW containing 61% P(3HB-co-16 mol% 4HB) was achieved [30]. Furthermore, by engineering the promoter driving the expression of orfZ gene, 100 g/L CDW containing 80% P(3HB-co-11 mol% 4HB) was obtained in unsterile fed-batch fermentation [31]. Finally, to synthesize P(3HB-co-4HB) using only glucose, the orfZ gene and ogdA encoding 2-oxo-glutarate dehydrogenase, sucD and 4hbd genes were introduced in H. bluephagenesis TD01 (Figure 3) [32]. This would direct succinate semialdehyde derived from 2-oxoglutarate and succinyl-CoA in the TCA cycle towards 4HB-CoA generation. Simultaneously, gabD genes were deleted to avoid the conversion of succinate semialdehyde to succinate [32]. The resulting strain was able to produce 26.3 g/L CDW containing 60.5% P(3HB-co-17 mol% 4HB) in 7-L bioreactor after 60 h of fed-batch fermentation under unsterile conditions. Moreover, by controlling the concentration of residual glucose in the culture, the 4HB fraction varied from 13.4 to 24.9 mol%. Thus, H. bluephagenesis TD01 holds high promise as an efficient industrial producer of P(3HB-co-4HB) polymer.
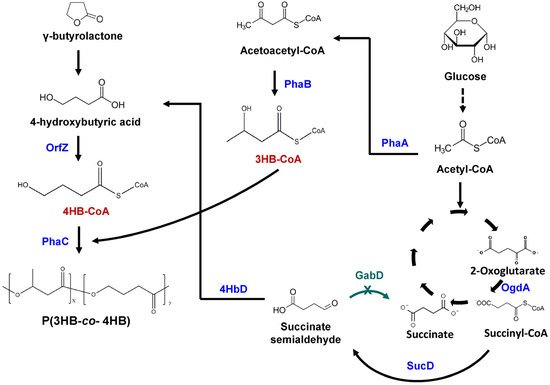
Figure 3. Metabolic engineering of Halomonas bluephagenesis TD01 to synthesize P (3HB-co-4HB). When using γ-butyrolactone as 4HB precursor, 4-hydroxybutyric acid is converted to 4HB-CoA by OrfZ. 3HB-CoA is derived from acetyl-CoA under the action of PhaA and PhaB. When using glucose as the sole substrate, it is converted to succinyl-CoA via glycolytic pathway and TCA cycle. OgdA catalyzes the conversion of 2-oxoglutarate to succinyl-CoA which is further converted to succinate semialdehyde by SucD. Deletion of gabD genes prevented the conversion of succinate semialdehyde to succinate. Next, succinate semialdehyde is converted to 4-hydroxybutyric acid by 4HbD. Under the action of PhaC, 3HB-CoA and 4HB-CoA are polymerized to form P(3HB-co-4HB). OgdA, 2-oxoglutarate dehydrogenase; SucD, succinate semialdehyde dehydrogenase; GabD, succinate semialdehyde dehydrogenase; 4HbD, 4-hydroxybutyrate dehydrogenase; OrfZ, CoA transferase; PhaA, β-ketothiolase; PhaB, acetoacetyl-CoA reductase; PhaC, PHA synthase.
References
- Accinelli, C.; Saccà, M.L.; Mencarelli, M.; Vicari, A. Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 2012, 89, 136–143.
- Kumar, V.; Kumar, S.; Singh, D. Microbial polyhydroxyalkanoates from extreme niches: Bioprospection status, opportunities and challenges. Int. J. Biol. Macromol. 2020, 147, 1255–1267.
- Reddy, C.S.K.; Ghai, R.; Kalia, V. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146.
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578.
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96.
- Huang, P.; Okoshi, T.; Mizuno, S.; Hiroe, A.; Tsuge, T. Gas chromatography-mass spectrometry-based monomer composition analysis of medium-chain-length polyhydroxyalkanoates biosynthesized by Pseudomonas spp. Biosci. Biotechnol. Biochem. 2018, 82, 1615–1623.
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908.
- Barbosa, J.L.; Perin, G.B.; Felisberti, M.I. Plasticization of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with an Oligomeric Polyester: Miscibility and Effect of the Microstructure and Plasticizer Distribution on Thermal and Mechanical Properties. ACS Omega 2021, 6, 3278–3290.
- Han, J.; Wu, L.P.; Liu, X.B.; Hou, J.; Zhao, L.L.; Chen, J.Y.; Zhao, D.H.; Xiang, H. Biodegradation and biocompatibility of haloarchaea-produced poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymers. Biomaterials 2017, 139, 172–186.
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly (4-hydroxybutyrate): Current state and perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257.
- Martin, D.P.; Badhwar, A.; Shah, D.V.; Rizk, S.; Eldridge, S.N.; Gagne, D.H.; Ganatra, A.; Darois, R.E.; Williams, S.F.; Tai, H.C.; et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J. Surg. Res. 2013, 184, 766–773.
- Le Meur, S.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Poly (4-hydroxybutyrate) (P4HB) production in recombinant Escherichia coli: P4HB synthesis is uncoupled with cell growth. Microb. Cell Fact. 2013, 12, 1–11.
- Zhou, X.Y.; Yuan, X.X.; Shi, Z.Y.; Meng, D.C.; Jiang, W.J.; Wu, L.P.; Chen, J.C.; Chen, G.Q. Hyperproduction of poly (4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb. Cell Fact. 2012, 11, 1–8.
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105.
- Wu, Q.; Wang, Y.; Chen, G.Q. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 1–12.
- Van Rooijen, M.M.J.; Jairam, A.P.; Tollens, T.; Jørgensen, L.N.; de Vries Reilingh, T.S.; Piessen, G.; Köckerling, F.; Miserez, M.; Windsor, A.C.J.; Berrevoet, F.; et al. A post-market, prospective, multi-center, single-arm clinical investigation of Phasix™ mesh for VHWG grade 3 midline incisional hernia repair: A research protocol. BMC Surgery 2018, 18, 1–9.
- Scott, J.R.; Deeken, C.R.; Martindale, R.G.; Rosen, M.J. Evaluation of a fully absorbable poly-4-hydroxybutyrate/absorbable barrier composite mesh in a porcine model of ventral hernia repair. Surg. Endosc. 2016, 30, 3691–3701.
- Molina, C.P.; Hussey, G.S.; Liu, A.; Eriksson, J.; D’Angelo, W.A.; Badylak, S.F. Role of 4-hydroxybutyrate in increased resistance to surgical site infections associated with surgical meshes. Biomaterials 2021, 267, 120493.
- Dvorin, E.L.; Wylie-Sears, J.; Kaushal, S.; Martin, D.P.; Bischoff, J. Quantitative Evaluation of Endothelial Progenitors and Cardiac Valve Endothelial Cells: Proliferation and Differentiation on Poly-glycolic acid/Poly-4-hydroxybutyrate Scaffold in Response to Vascular Endothelial Growth Factor and Transforming Growth Factor β1. Tissue Eng. 2003, 9, 487–493.
- Verhorstert, K.W.; Guler, Z.; de Boer, L.; Riool, M.; Roovers, J.P.W.; Zaat, S.A. In Vitro Bacterial Adhesion and Biofilm Formation on Fully Absorbable Poly-4-hydroxybutyrate and Nonabsorbable Polypropylene Pelvic Floor Implants. ACS Appl. Mater. Interfaces 2020, 12, 53646–53653.
- Deeken, C.R.; Matthews, B.D. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate—PHASIX Mesh) in a porcine model of hernia repair. Int. Sch. Res. Not. 2013, 2013, 1–12.
- Ramachandran, H.; Iqbal, N.M.; Sipaut, C.S.; Abdullah, A.A.A. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) terpolymer with various monomer compositions by Cupriavidus sp. USMAA2-4. Appl. Biochem. Biotechnol. 2011, 164, 867–877.
- Choi, M.H.; Song, J.J.; Yoon, S.C. Biosynthesis of copolyesters by Hydrogenophaga pseudoflava from various lactones. Can. J. Microbiol. 1995, 41, 60–67.
- Söhling, B.; Gottschalk, G. Molecular analysis of the anaerobic succinate degradation pathway in Clostridium kluyveri. J. Bacteriol. 1996, 178, 871–880.
- Doi, Y.; Segawa, A.; Kunioka, M. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 1990, 12, 106–111.
- Madden, L.A.; Anderson, A.J.; Asrar, J.; Berger, P.; Garrett, P. Production and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) synthesized by Ralstonia eutropha in fed-batch cultures. Polymer 2000, 41, 3499–3505.
- Chanprateep, S.; Kulpreecha, S. Production and characterization of biodegradable terpolymer poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) by Alcaligenes sp. A-04. J. Biosci. Bioeng. 2006, 101, 51–56.
- Sedlacek, P.; Pernicova, I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the newly isolated bacterium Aneurinibacillus sp. H1 as an auspicious thermophilic producer of various polyhydroxyalkanoates (PHA) copolymers–2. Material study on the produced copolymers. Polymers 2020, 12, 1298.
- Hein, S.; Söhling, B.; Gottschalk, G.; Steinbüchel, A. Biosynthesis of poly (4-hydroxybutyric acid) by recombinant strains of Escherichia coli. FEMS Microbiol. Lett. 1997, 153, 411–418.
- Chen, X.; Yin, J.; Ye, J.; Zhang, H.; Che, X.; Ma, Y.; Li, M.; Wu, L.P.; Chen, G.Q. Engineering Halomonas bluephagenesis TD01 for non-sterile production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Bioresour. Technol. 2017, 244, 534–541.
- Shen, R.; Yin, J.; Ye, J.W.; Xiang, R.J.; Ning, Z.Y.; Huang, W.Z.; Chen, G.Q. Promoter engineering for enhanced P (3HB-co-4HB) production by Halomonas bluephagenesis. ACS Synth. Biol. 2018, 7, 1897–1906.
- Ye, J.; Hu, D.; Che, X.; Jiang, X.; Li, T.; Chen, J.; Zhang, H.M.; Chen, G.Q. Engineering of Halomonas bluephagenesis for low cost production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 2018, 47, 143–152.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
09 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




