Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ANA KAREN MENDOZA MARTINEZ | + 1360 word(s) | 1360 | 2021-11-23 05:17:10 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1360 | 2021-12-06 01:45:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mendoza Martinez, A.K. Tumor Microenvironment of Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/16734 (accessed on 07 February 2026).
Mendoza Martinez AK. Tumor Microenvironment of Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/16734. Accessed February 07, 2026.
Mendoza Martinez, Ana Karen. "Tumor Microenvironment of Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/16734 (accessed February 07, 2026).
Mendoza Martinez, A.K. (2021, December 04). Tumor Microenvironment of Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/16734
Mendoza Martinez, Ana Karen. "Tumor Microenvironment of Ovarian Cancer." Encyclopedia. Web. 04 December, 2021.
Copy Citation
Ovarian cancer (OvCa) is one of the leading causes of gynecologic malignancies. Despite treatment with surgery and chemotherapy, OvCa disseminates and recurs frequently, reducing the survival rate for patients. There is an urgent need to develop more effective treatment options for women diagnosed with OvCa. The tumor microenvironment (TME) is a key driver of disease progression, metastasis and resistance to treatment. For this reason, 3D models have been designed to represent this specific niche and allow more realistic cell behaviors compared to conventional 2D approaches.
ovarian cancer
tumor microenvironment
peptides
biomaterial
self-assembly
mechanical properties
extracellular matrix
3D models
1. Introduction
Ovarian cancer (OvCa) is one of the leading causes of cancer-related deaths among women, largely due to its late diagnosis, high metastatic potential and resistance to chemotherapy [1][2]. Due to the lack of specific clinical symptoms and early diagnosis, most patients are diagnosed at an advanced stage (FIGO stages III and IV) with intra-abdominal metastasis and the formation of ascites [2][3][4].
Distant metastasis occurs due to the shedding of cancer cells from the primary tumor, as spheroids or single cells, into the peritoneal cavity and the formation of ascites. Ascites, or tumor fluid, contains cellular components, cytokines, growth factors and other secreted molecules that support tumor cell proliferation and migration. This rich tumor-promoting microenvironment supports cancer cells to overcome apoptosis and inhibits the response to chemotherapy [5]. Tumor cells or spheroids in the ascitic microenvironment settle onto the mesothelial lining of the peritoneum, disaggregate and invade into the extracellular matrix (ECM) to form metastatic lesions [6][7].
In order to better understand the progression of OvCa, and to develop new and more effective therapeutic strategies, animal and 3D in vitro approaches have been engineered to recapitulate the unique TME of OvCa. Modeling OvCa is immensely complex due to diverse cell populations, pathological and genetic complexity (heterogeneity) and unknown recurrence mechanisms. If designed and implemented successfully, these approaches have the potential to improve cancer diagnosis and subsequent treatment.
2. Components of the Ovarian TME
The TME is comprised of cancer and stromal cells, signaling molecules, exosomes, and the ECM, and has long been implicated in the progression, metastasis and resistance to treatment [8][9]. Cellular and acellular components vary depending on the disease site, for example, the primary tumor site, ascites and secondary tumor site, which modulate the signals received by the cancer cells as summarized in Figure 1 [10].
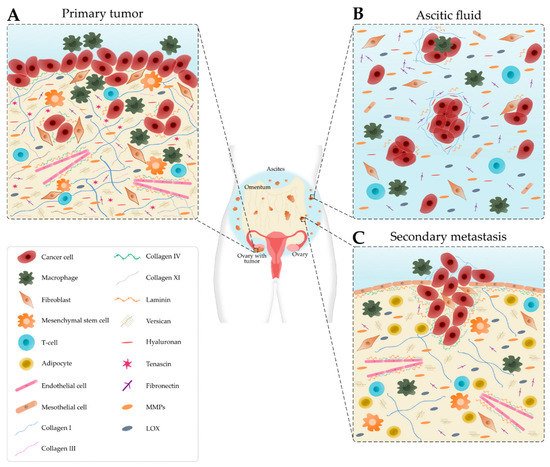
Figure 1. Schematic representation of the main cellular components and extracellular matrix composition of the ovarian tumor microenvironment depending on the disease site. (A) In the primary tumor, cancer cells recruit tumor-associated macrophages, cancer-associated fibroblasts, T-cells and endothelial cells. Many extracellular matrix components, such as fibronectin, hyaluronan, tenascin, versican, matrix metalloproteinases (MMPs) and lysyl oxidase (LOX) are upregulated. Collagen progressively remodels into thick fibrils and is randomly oriented. Laminin and collagen IV are underexpressed. (B) Detached single cells or spheroids are immersed in the ascitic fluid, which contains macrophages, fibroblasts, mesothelial cells and immune cells. Extracellular matrix components are found within the aggregated cells and the ascitic fluid. (C) Cancer cells settle onto the mesothelial lining to form secondary tumors that are rich in collagen. Laminin and collagen IV are overexpressed to promote metastasis. Levels of collagen I and III start to decrease.
2.1. Cellular Composition
OvCa cells are a diverse mix of cells with distinct properties and functions. Cancer stem cells (CSCs) represent a small sub-population that have properties of self-renewal, multi-lineage differentiation and resistance to anoikis [11]. CSCs contribute to tumor initiation, metastasis and resistance to treatment [12][13]. Several mechanisms within CSCs confer a survival advantage during treatment, including increased resistance to apoptosis, dormancy, the expression of ATP-binding cassette (ABC) transporters, the upregulation of aldehyde dehydrogenases (ALDHs), the response to DNA damage and epithelial-to-mesenchymal transition (EMT). As a result, CSCs lead to disease relapse by escaping treatment and repopulating the tumor with a heterogeneous and more aggressive population of cancer cells [14].
Within the stromal microenvironment, tumor-associated macrophages (TAMs) constitute the main population of immune cells in the primary tumor and ascites [15]. TAMs are not only key players in the implantation of cancer cells in the omentum, but also facilitate angiogenesis, metastasis and chemoresistance [15][16][17]. TAMs, together with myeloid-derived suppressor cells (MDSCs), contribute to the immune escape of cancer cells by hindering the cytotoxic activity of natural killer cells and cytotoxic T cells, inhibiting the maturation of dendritic cells (DCs) and recruiting regulatory T cells [15][18][19][20]. On the other hand, OvCa-derived exosomes can induce the polarization of macrophages in an M2-like phenotype and the apoptosis of DCs and lymphocytes [21].
OvCa cells reprogram stromal cells into a pro-tumoral phenotype, like for example, cancer-associated fibroblasts (CAFs). CAFs are reprogrammed fibroblasts that are crucial in the deposition and remodeling of the ECM through the secretion of MMPs and other secreted factors [22][23]. CAFs secrete pro-inflammatory cytokines (e.g., COX-2, CXL1, CCL5, CXC11 and IL-6) that induce EMT, cancer cell proliferation, invasion, chemoresistance and inhibit cancer cell apoptosis [22][24][25].
Mesenchymal stem cells (MSCs) can be converted to cancer-associated MSCs (CA-MSCs) and later differentiate into fibroblasts, osteocytes or adipocytes [26]. The upregulation of TGF-β in CA-MSCs increases the number of CSCs and chemoresistance [27][28]. Particularly, adipocytes located in the omentum attract cancer cells through the secretion of IL-8 and support cancer cell proliferation through fatty acids [29][30]. Adipocytes also secrete factors that enhance chemoresistance by activating the Akt pathway [30]. Elevated levels of VEGF secreted by tumor cells, TAMs, CAFs and adipocytes lead to angiogenesis, endothelial cell survival, proliferation, migration and vascular permeability [31].
2.2. Matrix Composition
One major constituent of the ovarian TME is the ECM, a meshwork of proteins (e.g., collagen, fibronectin, tenascin and laminin), glycosaminoglycans (e.g., hyaluronan), proteoglycans (e.g., versican) and remodeling enzymes (e.g., MMP2, MMP9 and LOX). The ECM provides both biomechanical and biochemical support for cancer cell proliferation and metastasis [32]. Continuous remodeling of the ECM, induced by cancer and stromal cells, changes its mechanical properties, thereby influencing disease progression and chemoresistance [33]. Additionally, cancer cells experience numerous mechanical stimuli within the ovarian TME, such as shear stress, compressive stress, tensile stress and stress relaxation, which directly affect their behavior and signaling pathways [34].
In normal ovarian tissue, collagen fibers are thin and long while in tumor stroma they are thicker and shorter fibrils perpendicularly aligned rather than parallel to the tumor boundary [35]. Elevated collagen deposition and remodeling promote tumor progression and drug resistance [32]. Collagen type I enhances the migration of multiple cancer cell lines due to increased directionality [36]. At early stages, collagen types I and III are widely distributed; however, the levels decrease as the disease progresses [37]. During OvCa progression, high levels of collagen type XI have an important role in cell invasiveness, cell proliferation and tumor formation [38]. Collagen type IV and laminin are often absent in benign ovarian surfaces and primary ovarian tumors. At later stages, the restoration of collagen type IV and laminin presence promotes the spread of OvCa cells to metastatic sites in the peritoneum [39][40].
Hyaluronan is a major component of the ECM that has been associated with metastatic progression and poor ovarian cancer outcomes [16][41]. High levels of hyaluronan were detected in metastatic lesions and primary tumors [42] and correlated with increased versican levels [32]. In OvCa, the interaction between hyaluronan and its cell surface receptor, CD44, facilitates cell adhesion, migration, tumor growth and peritoneal dissemination [41][42][43]. Increased expression of fibronectin was found in metastatic tumors and ascitic fluid. Fibronectin is an indicator of poor prognosis that contributes to the formation, adhesion and disaggregation of OvCa spheroids and promotes tumor migration, invasion and metastasis [32][44]. The stroma also contains tenascin-C that contributes to tumorigenesis and metastasis [45]. Tenascin-X is considered as potential biomarker of OvCa as it is highly overexpressed in tumorous tissues [46]. In addition, the downregulation of decorin and lumican are involved in cancer progression and aggressiveness, respectively [32]. As ECM-associated components, MMPs, mediate ECM remodeling and tumor development. Particularly, MMP-9 and MMP-2 facilitate cancer cell invasion via the degradation of collagen IV at the basement membrane [16]. Overexpression of LOX enhances the crosslinking of the ECM proteins resulting in increased matrix stiffness [32].
As outlined above, the bidirectional interactions between cancer cells and the TME are crucial in disease progression. For this reason, it is critical to understand the functions of cancer cells and how the TME regulates the surrounding stroma to promote disease progression.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A.; Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018, 68, 7-30, 10.3322/caac.21442.
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; KaramiNejadRanjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885-2898, 10.1038/s41388-018-0637-x.
- Lane, D.; Matte, I.; Garde-Granger, P.; Laplante, C.; Carignan, A.; Rancourt, C.; Piché, A.; Inflammation-regulating factors in ascites as predictive biomarkers of drug resistance and progression-free survival in serous epithelial ovarian cancers. BMC Cancer 2015, 15, 1-11, 10.1186/s12885-015-1511-7.
- Karnezis, A.N; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G.; The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nature Reviews Cancer 2017, 17, 65-74, 10.1038/nrc.2016.113.
- Kipps, E.; Tan, D.; Kaye, S.B.; Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nature Reviews Cancer 2013, 13, 273-282, 10.1038/nrc3432.
- Shield, K.; Ackland, L.; Ahmed, N.; Rice, G.; Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecologic Oncology 2009, 113, 143-148, 10.1016/j.ygyno.2008.11.032.
- Ahmed, N.; Thompson, E.W.; Quinn, M.; Epithelial–mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: An exception to the norm. Journal of Cellular Physiology 2007, 213, 581-588, 10.1002/jcp.21240.
- Park, K.M.; Lewis, D.; Gerecht, S.; Bioinspired Hydrogels to Engineer Cancer Microenvironments. Annual Review of Biomedical Engineering 2017, 19, 109-133, 10.1146/annurev-bioeng-071516-044619.
- Brown, Y.; Hua, S.; Tanwar, P.S.; Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. The International Journal of Biochemistry & Cell Biology 2019, 109, 90-104, 10.1016/j.biocel.2019.02.002.
- Shih, A.J.; Menzin, A.; Whyte, J.; Lovecchio, J.; Liew, A.; Khalili, H.; Bhuiya, T.; Gregersen, P.K.; Lee, A.T.; Correction: Identification of grade and origin specific cell populations in serous epithelial ovarian cancer by single cell RNA-seq. PLoS ONE 2018, 13, e0208778, 10.1371/journal.pone.0208778.
- Barbato, L.; Bocchetti, M.; Di Biase, A.; Regad, T.; Cancer Stem Cells and Targeting Strategies. Cells 2019, 8, 926, 10.3390/cells8080926.
- Yu, Z.; Pestell, T.G.; Lisanti, M.; Pestell, R.G.; Cancer stem cells. The International Journal of Biochemistry & Cell Biology 2012, 44, 2144-2151, 10.1016/j.biocel.2012.08.022.
- Foster, R.; Buckanovich, R.J.; Rueda, B.R.; Ovarian cancer stem cells: Working towards the root of stemness. Cancer Letters 2013, 338, 147-157, 10.1016/j.canlet.2012.10.023.
- Zhao, J.; Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacology & Therapeutics 2016, 160, 145-158, 10.1016/j.pharmthera.2016.02.008.
- Nowak, M.; Klink, M.; The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299, 10.3390/cells9051299.
- Luo, Z.; Wang, Q.; Lau, W.B.; Lau, B.; Xu, L.; Zhao, L.; Yang, H.; Feng, M.; Xuan, Y.; Yang, Y.; et al. Tumor microenvironment: The culprit for ovarian cancer metastasis?. Cancer Letters 2016, 377, 174-182, 10.1016/j.canlet.2016.04.038.
- Ge, Z.; Ding, S.; The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Frontiers in Oncology 2020, 10, 2404, 10.3389/fonc.2020.590941.
- Hansen, J.M.; Coleman, RL.; Sood, A.K.; Targeting the tumour microenvironment in ovarian cancer. European Journal of Cancer 2016, 56, 131-143, 10.1016/j.ejca.2015.12.016.
- Salas-Benito, D.; Vercher, E.; Conde, E.; Glez-Vaz, J.; Tamayo, I.; Hervas-Stubbs, S.; Inflammation and immunity in ovarian cancer. European Journal of Cancer Supplements 2020, 15, 56-66, 10.1016/j.ejcsup.2019.12.002.
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K.; Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in Cancer Biology 2012, 22, 275-281, 10.1016/j.semcancer.2012.01.011.
- Li, X.; Liu, Y.; Zheng, S.; Zhang, T.; Wu, J.; Sun, Y.; Zhang, J.; Liu, G.; Role of exosomes in the immune microenvironment of ovarian cancer (Review). Oncology Letters 2021, 21, 1-17, 10.3892/ol.2021.12638.
- Dasari, S.; Fang, Y.; Mitra, A.K.; Cancer Associated Fibroblasts: Naughty Neighbors That Drive Ovarian Cancer Progression. Cancers 2018, 10, 406, 10.3390/cancers10110406.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nature Reviews Cancer 2020, 20, 174-186, 10.1038/s41568-019-0238-1.
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C.; The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 10, 242, 10.3390/cancers10080242.
- Han, Q.; Huang, B.; Huang, Z.; Cai, J.; Gong, L.; Zhang, Y.; Jiang, J.; Dong, W.; Wang, Z.; Tumor cell‑fibroblast heterotypic aggregates in malignant ascites of patients with ovarian cancer. International Journal of Molecular Medicine 2019, 44, 2245-2255, 10.3892/ijmm.2019.4361.
- Coffman, L.G.; Choi, Y.-J.; McLean, K.; Allen, B.L.; di Magliano, M.P.; Buckanovich, R.J.; Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget 2016, 7, 6916-6932, 10.18632/oncotarget.6870.
- McLean, K.; Gong, Y.; Choi, Y.; Deng, N.; Yang, K.; Bai, S.; Cabrera, L.; Keller, E.; McCauley, L.; Cho, K.; et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. Journal of Clinical Investigation 2011, 121, 3206-3219, 10.1172/jci45273.
- Coffman, L.G.; Pearson, A.; Frisbie, L.G.; Freeman, Z.; Christie, E.; Bowtell, D.D.; Buckanovich, R.J.; Ovarian Carcinoma‐Associated Mesenchymal Stem Cells Arise from Tissue‐Specific Normal Stroma. STEM CELLS 2018, 37, 257-269, 10.1002/stem.2932.
- Nieman, K.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine 2011, 17, 1498-1503, 10.1038/nm.2492.
- Yang, J.; Zaman, M.M.; Vlasakov, I.; Roy, R.; Huang, L.; Martin, C.R.; Freedman, S.D.; Serhan, C.N.; Moses, M.A.; Adipocytes promote ovarian cancer chemoresistance. Scientific Reports 2019, 9, 1-12, 10.1038/s41598-019-49649-1.
- Moghaddam, S.M.; Amini, A.; Morris, D.L.; Pourgholami, M.H.; Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer and Metastasis Reviews 2011, 31, 143-162, 10.1007/s10555-011-9337-5.
- Echo, A.; Howell, V.M.; Colvin, E.K.; The Extracellular Matrix in Epithelial Ovarian Cancer – A Piece of a Puzzle. Frontiers in Oncology 2015, 5, 245, 10.3389/fonc.2015.00245.
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K.; The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Scientific Reports 2018, 8, 1-20, 10.1038/s41598-018-25589-0.
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T.; The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008, 10.3390/cancers11071008.
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J.; Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Frontiers in Cell and Developmental Biology 2021, 9, 1-13, 10.3389/fcell.2021.718834.
- Liu, M.; Zhang, X.; Long, C.; Xu, H.; Cheng, X.; Chang, J.; Zhang, C.; Zhang, C.; Wang, X.; Collagen-based three-dimensional culture microenvironment promotes epithelial to mesenchymal transition and drug resistance of human ovarian cancerin vitro. RSC Advances 2018, 8, 8910-8919, 10.1039/c7ra13742g.
- Pearce, O.; Delaine-Smith, R.M.; Maniati, E.; Nichols, S.; Wang, J.; Böhm, S.; Rajeeve, V.; Ullah, D.; Chakravarty, P.; Jones, R.R.; et al. Deconstruction of a Metastatic Tumor Microenvironment Reveals a Common Matrix Response in Human Cancers. Cancer Discovery 2018, 8, 304-319, 10.1158/2159-8290.cd-17-0284.
- Wu, Y.-H.; Chang, T.-H.; Huang, Y.-F.; Huang, H.-D.; Chou, C.-Y.; COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 2014, 33, 3432-3440, 10.1038/onc.2013.307.
- Ajeti, V.; Lara-Santiago, J.; Alkmin, S.; Campagnola, P.J.; Ovarian and Breast Cancer Migration Dynamics on Laminin and Fibronectin Bi-directional Gradient Fibers Fabricated via Multiphoton Excited Photochemistry. Cellular and Molecular Bioengineering 2017, 10, 295-311, 10.1007/s12195-017-0492-9.
- Bar, J.K.; Grelewski, P.; Popiela, A.; Noga, L.; Rabczyñski, J.; Type IV collagen and CD44v6 expression in benign, malignant primary and metastatic ovarian tumors: correlation with Ki-67 and p53 immunoreactivity. Gynecologic Oncology 2004, 95, 23-31, 10.1016/j.ygyno.2004.06.046.
- Ricciardelli, C.; Rodgers, R.J.; Extracellular Matrix of Ovarian Tumors. Seminars in Reproductive Medicine 2006, 24, 270-282, 10.1055/s-2006-948556.
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma V.M.; High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000, 60, 150-155.
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W.; The biology and role of CD44 in cancer progression: therapeutic implications. Journal of Hematology & Oncology 2018, 11, 1-23, 10.1186/s13045-018-0605-5.
- Kenny, H.A.; Chiang, C.-Y.; White, E.A.; Schryver, E.M.; Habis, M.; Romero, I.; Ladanyi, A.; Penicka, C.V.; George, J.; Matlin, K.; et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. Journal of Clinical Investigation 2014, 124, 4614-4628, 10.1172/jci74778.
- Didem, T.; Faruk, T.; Senem, K.; Derya, D.; Murat, S.; Murat, G.; Oznur, K.; Clinical significance of serum tenascin-c levels in epithelial ovarian cancer. Tumor Biology 2014, 35, 6777-6782, 10.1007/s13277-014-1923-z.
- Kramer, M.; Pierredon, S.; Ribaux, P.; Tille, J.-C.; Petignat, P.; Cohen, M.; Secretome Identifies Tenascin-X as a Potent Marker of Ovarian Cancer. BioMed Research International 2015, 2015, 1-9, 10.1155/2015/208017.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




