Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Massa | + 2039 word(s) | 2039 | 2021-12-03 07:34:06 | | | |
| 2 | Camila Xu | + 83 word(s) | 2122 | 2021-12-03 09:32:23 | | | | |
| 3 | Camila Xu | + 83 word(s) | 2122 | 2021-12-03 09:33:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Massa, A. Arabinogalactan (AG) and Hyaluronic Acid (HA). Encyclopedia. Available online: https://encyclopedia.pub/entry/16711 (accessed on 07 February 2026).
Massa A. Arabinogalactan (AG) and Hyaluronic Acid (HA). Encyclopedia. Available at: https://encyclopedia.pub/entry/16711. Accessed February 07, 2026.
Massa, Antonio. "Arabinogalactan (AG) and Hyaluronic Acid (HA)" Encyclopedia, https://encyclopedia.pub/entry/16711 (accessed February 07, 2026).
Massa, A. (2021, December 03). Arabinogalactan (AG) and Hyaluronic Acid (HA). In Encyclopedia. https://encyclopedia.pub/entry/16711
Massa, Antonio. "Arabinogalactan (AG) and Hyaluronic Acid (HA)." Encyclopedia. Web. 03 December, 2021.
Copy Citation
The properties of mixtures of two polysaccharides, arabinogalactan (AG) and hyaluronic acid (HA), were investigated in solution by the measurement of diffusion coefficients D of water protons by DOSY (Diffusion Ordered SpectroscopY), by the determination of viscosity and by the investigation of the affinity of a small molecule molecular probe versus AG/HA mixtures in the presence of bovine submaxillary mucin (BSM) by 1HNMR spectroscopy.
polysaccharides
hyaluronic acid-arabinogalactan mixtures
diffusion ordered spectroscopY (DOSY)
1. Introduction
Hydrogels formed by chemically cross-linked hyaluronic acid (HA) are widely used in the formulation of artificial tears for the treatment of dry eye syndrome [1][2][3][4][5][6][7]. Natural HA is a linear anionic polysaccharide consisting of alternating units of N-acetyl-D-glucosamine and sodium-D-glucuronate groups (Figure 1). With a molecular weight that can reach several millions of Dalton, HA is involved in numerous biological functions [8][9][10][11][12]. It is soluble in water and has a high degree of functionalization and charge density. Its solutions show high viscosity; in solution, it usually arranges in a 3D structure characterized by intramolecular hydrogen bonding [13][14]. This peculiarity also drives its physical–chemical interactions with other molecules. All these properties, as well as its biodegradability and immune neutrality, make HA an optimal biomaterial for wound healing applications and tissue engineering [14][15], in particular, in the regeneration of cartilage [16] and teeth structure [17].
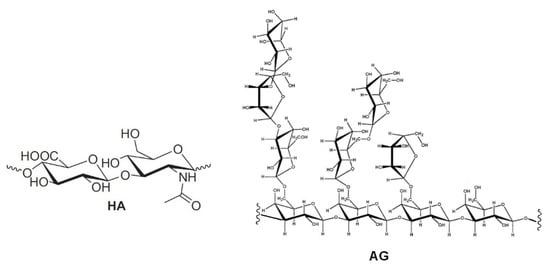
Figure 1. Repetitive unit of hyaluronic acid (HA) and a structural fragment of arabinogalactan (AG).
In order to overcome the issues related to chemical cross-linking and modifications of natural HA [5][6][7][8], the development of new formulations, formed by mixtures of different polysaccharides, has been proposed [18][19]. In particular, enhanced mucoadhesive properties have been reported for mixtures of (HA) and tamarind-seed polysaccharide (TSP), a cellulose-like polysaccharide with a high degree of branched glycosyl substitution [18][19]. This mixture is a candidate for new formulations of artificial eye drops. A new artificial eye drop formed by the combination of hyaluronic acid (HA) with another polysaccharide, arabinogalactan (AG), has been recently proposed by Silvani and co-workers [20]. Notably, this mixture synergistically decreases the xanthine oxidoreductase (XOR) activity, inhibiting UA (uric acid) and ROS (reactive oxygen species) formation, and therefore it may contribute to the treatment of the dry eye syndrome, reducing irritation and related pathological conditions. AG is a natural polysaccharide formed of arabinose and galactose in a ratio of 1:6 and a molecular weight ranging between 10,000 and 120,000 Da [21]. AG is mostly extracted from the Larch tree (Larix occidentalis L. decidua) but it is abundant in a large variety of plants. Due to the broad array of species, the available AGs show a very wide range of biological properties and documented activities such as the improvement of vascular permeability [22], the support of digestive health by improving intestinal microflora [23][24][25], the enhancement of the immune function [21][22] and a significant in vitro stimulation of dermal fibroblast activity and proliferation [26]. Besides all these properties, AG is also extensively used for treating skin burns and wound-healing in middle and South America [26]. The AG wound-healing property is probably due to the combination of its anti-inflammatory [27], cicatrizing [28] and antimicrobial effects [29]. AG has been approved by the FDA for human consumption in large quantities [30], thus it represents an effective natural alternative to be used for both wound-healing and the reduction of ROS in different matrixes. AG has a high morphological freedom and it has flexible branches with exposed hydroxyl groups (lateral groups) (Figure 1); this peculiarity may play a key role in the interaction with other molecules and polysaccharides.
Nevertheless, to our knowledge, articles reporting physical–chemical properties of mixtures of AG and HA are still lacking. In this context, as part of our interest in the analysis of biomolecules [31], the aim of the present study is the investigation of mixtures of arabinogalactan (AG) and hyaluronic acid (HA) by viscosity measurements and NMR spectroscopy. This investigation can be very useful in highlighting new properties for the development of new formulations of the two polysaccharides for pharmaceuticals and cosmetic applications. The determination of possible synergic effects between different polysaccharides in solution is often challenging and there is a need for simple and reliable methods that can be used to highlight interactions at molecular level. For this purpose, solution rheology and Nuclear Magnetic Resonance (NMR) are particularly useful since the analysis is very simple and reliable, while no sample derivatization or pre-treatment is required [7][12][18][19]. As water is the main component of polysaccharide-based eye drops, studying the behavior of solutions at macroscopic and microscopic levels might show correlations between different polysaccharides, or provide information on their physicochemical properties [7][12][18][19]. In particular, DOSY (Diffusion Ordered SpectroscopY) determines the diffusion coefficient of H2O among other molecules and, therefore, this measure can be correlated to the water incorporated in the polymers and to the interactions between polymers of different natures [12]. Additional information about interactions between polysaccharides can be obtained using small molecule molecular probes, as reported in a recent investigation about the properties of solutions of HA and TSP [18][19]. In particular, the formation of stable supramolecular aggregates and enhanced mucoadhesive properties of polysaccharide mixtures have been correlated, by NMR spectroscopic methods in a four-component solution, to their affinity with the anti-inflammatory drug diclofenac sodium salt (DS, Figure 2), a small molecule molecular probe, with respect to bovine submaxillary mucin (BSM) [18][19]. In this case, the interaction between the two polysaccharides was sufficiently effective to perturb the known interaction between DS and mucin and, consequently, the NMR signals of the molecular probe itself.
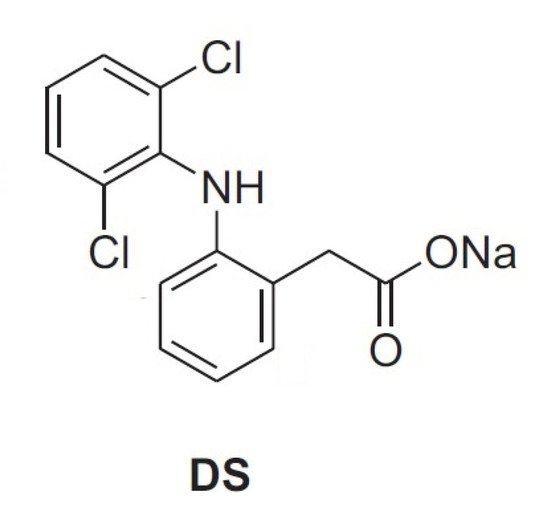
Figure 2. Diclofenac sodium salt.
2. Diffusion Coefficients of Water in AG/HA Solutions
Water proton diffusion coefficients D determined by DOSY (Diffusion Ordered SpectroscopY) have been recently utilized by Wende et al. [12] in the characterization of crosslinked HA hydrogels in 90/10 H2O/D2O solution. Therefore, we thought to investigate the properties of AG/HA mixtures at different ratios, measuring D of water protons in H2O/D2O at 90/10 ratio by DOSY using a 400 MHz spectrometer at 25.0 ± 0.1 °C (Table 1). In the preliminary experiments, we soon found that the value of reference D (Entry 1) was in accordance with that reported by Wende [12]. Then, we proceeded to analyse solutions of the two polysaccharides. The value of D at 1/1 ratio AG/HA (Entry 2) was close to that of reference D of water protons. However, considering the standard errors, a sharp comparison cannot be performed. A further increase of AG concentration shows, however, a clear trend, indicating a progressive decrease in the mobility of water (Table 1, Entries 3–5).
Table 1. Diffusion coefficients D of water protons in D2O/H2O = 10/90.
| Entry | Mixture (Total Conc. Polysaccharides) | Diffusion Coefficient H2O (×10−9 m2/s) a |
|---|---|---|
| 1 | Reference in D2O/H2O (10/90) | 2.07 ± 0.01 |
| 2 | AG/HA 1:1 (3.0 mg/mL) | 2.09 ± 0.02 |
| 3 | AG/HA 2:1 (4.5 mg/mL) | 2.07 ± 0.02 |
| 4 | AG/HA 3:1 (6.0 mg/mL) | 2.04 ± 0.02 |
| 5 | AG/HA 4:1 (7.5 mg/mL) | 1.99 ± 0.01 |
a Experiments performed on the same samples were run in duplicates.
As observed by Wende and co-workers [12], mono-component exponential fitting was found to give a good fit for most of the samples and used on all measurements to avoid overfitting. Relatively high standard errors were detected [12], comparable to those obtained by us, but, to the best of our knowledge, an investigation about the effect on D accuracy of H2O/D2O at different ratios has never been reported.
Therefore, we decided to determine diffusion coefficients D by DOSY using a 400 MHz spectrometer of H2O (0.1%) in large excess of D2O (99.9%) of solutions of AG and HA, at the same ratios and concentrations, for comparison. Solutions of solely AG or HA were also analyzed (Table 2). The value of reference D of water protons was significantly lower (Table 2, entry 1) than that previously determined (Table 1, entry 1). The presence of a larger amount of D2O and rapid proton/deuterium exchange would explain the reduced water mobility and therefore the reduced value of D. Since the diffusion coefficient is an average measure of bound and unbound water and the H2O/HDO ratio, the reduced mobility of water should be referred mainly to as HDO, which is the main species when an excess of D2O is present. The mass effect and increased hydrogen-bond strength cause the observed decrease of D with respect to those reported in Table 1 [32].
Table 2. Diffusion coefficients D of water protons in 99.9% D2O.
| Entry | Mixture (Total Conc. Carbohydrate) | Diffusion Coefficient H2O (×10−9 m2/s) a |
|---|---|---|
| 1 | Reference in D2O 99.9% | 1.703 ± 0.007 |
| 2 | AG (3.0 mg/mL) | 1.666 ± 0.005 |
| 3 | HA (3.0 mg/mL) | 1.658 ± 0.005 |
| 4 | AG/HA 1:1 (3.0 mg/mL) | 1.675 ± 0.006 |
| 5 | AG/HA 2:1 (4.5 mg/mL) | 1.666 ± 0.009 |
| 6 | AG/HA 3:1 (6.0 mg/mL) | 1.656 ± 0.005 |
| 7 | AG/HA 4:1 (7.5 mg/mL) | 1.648 ± 0.005 |
a Experiments performed on the same samples were run in duplicates.
D of water protons (0.1%) in D2O (99.9%) at 25.0 ± 0.1 °C was also determined for comparison at 600 MHz spectrometer obtaining a value of 1.714 × 10−9 m2/s, which is in accord with the one, within the experimental error, found at 400 MHz spectrometer (Table 2, entry 1). Encouraged by these preliminary results, we proceeded with an analysis of mixtures of AG/HA at different ratios. For all the tested mixtures, a lower value of D of water protons was detected with respect to reference D of water (Entry 1), indicating a progressive reduced mobility of water at the increase of AG concentration. A slight increase of water protons D value was detected at the ratio AG/HA = 1/1 (3.0 mg/mL total concentration, Table 2, entry 4) with respect to solutions of solely AG or HA at the same concentration (Table 2, entries 2 and 3), probably indicating that the slightly increased mobility of water could be due to the interactions between the two polysaccharides, and the formed aggregates tend to exclude water molecules which are more mobile. Then, a further increase of the AG/HA ratio and concentration (Table 2), leads to a progressive reduced mobility of water protons as previously determined (Table 1). In all the cases lower standard errors were detected (see Table 1 for comparison). The two series of data in Table 1 and Table 2 clearly indicate that the mobility of water is reduced increasing the concentration of AG. Probably, water molecules are entrapped and complexed by carboxylate and hydroxyl groups mainly via hydrogen bonds and van der Waals interactions, causing a macroscopic retainment of a higher amount of water and a progressive decrease in its mobility. These aspects can be of high importance because they can lead to new formulations for pharmaceuticals and cosmetics applications that show better hydrating power and higher retention of water increasing of AG concentration.
However, the decrease in the diffusion coefficient observed at the increase of the total concentration of AG could be ascribed to eventual increase of viscosity. Viscosity greatly affects diffusion of molecules and the diffusion coefficient D determined by DOSY experiments [14][33][34]. Therefore, the determination of viscosity of D2O solutions of the mixture of polysaccharides was performed for a better comprehension of the phenomena herein highlighted (see next section).
3. Determination of Viscosity on D2O 99.9% Solutions
The viscosity was determined on D2O solutions of AG/HA mixtures (Figure 1 and Table 3), because D2O and H2O have different viscosity [35]. The trends of viscosity versus the shear rate for the solely AG, HA and their mixtures is shown in Figure 3. Viscosity values at three different shear rates are listed in Table 3. First of all, the analysis of the curves highlighted an interesting phenomenon, a progressive decrease of the viscosity increasing the total concentration of AG at any shear rate (Figure 3). The correlation of the data in Table 2 with the viscosity measurements (Figure 3) shows lower diffusion coefficients at lower viscosity. As reported in the literature, the increase of the molecular weight and concentration of hyaluronic acid in polymer solutions leads to the reinforcement of the three-dimensional network of the polymer, and consequently an increased viscosity [14]. Favored by HA-AG interactions, the decrease of viscosity and the decrease of water mobility observed at the increase of AG concentration can be ascribed to reduction of molecular entanglements and intermolecular interactions between HA chains, and to the strengthening interactions with water. These outcomes have a great practical appeal because an efficient manipulation of the rheological behavior of HA solutions can be achieved when combined with AG. In perspective, these properties can allow a wider use of the two polysaccharides not only in eye-drops preparations, but also as a mix for cosmetics and medical device applications, when decreased viscosity combined with increased hydrating power are required.
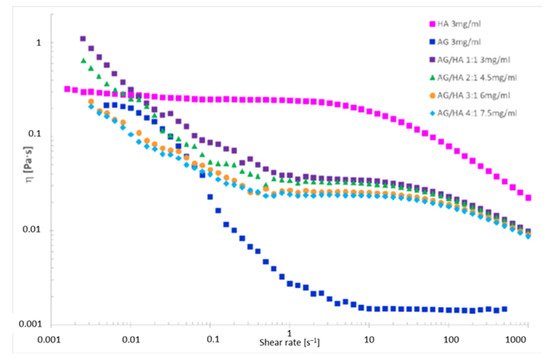
Figure 3. Viscosity vs. the shear rate for polysaccharide solutions in 99.9% D2O.
Table 3. Viscosity values for three different shear rates.
| Sample | η at 0.1 s−1 [mPa·s] |
η at 1 s−1 [mPa·s] |
η at 10 s−1 [mPa·s] |
|---|---|---|---|
| HA 3 mg/mL | 250.14 | 243.69 | 186.97 |
| AG 3 mg/mL | 22.90 | 2.73 | 1.48 |
| AG/HA 1:1 3 mg/mL | 86.69 | 38.87 | 34.04 |
| AG/HA 2:1 4.5 mg/mL | 53.91 | 34.43 | 31.66 |
| AG/HA 3:1 6 mg/mL | 44.60 | 26.93 | 25.48 |
| AG/HA 4:1 7.5 mg/mL | 39.81 | 24.29 | 23.46 |
The analysis of the viscosity curve of HA solution shows two trends:
1. Shear rate < 1 s−1 Newtonian behavior. The entanglements untangle and entangle again, thus viscosity is not affected by the shear rate.
2. Shear rate > 1 s−1 pseudoplastic behavior. The entanglements untangle and the chains orient themselves toward the shear direction, the gel deconstructs, the layers become thinner, and the viscosity drops down.
References
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of Sodium Hyaluronate Eyedrops in the Treatment of Dry Eye. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112.
- Stuart, J.C.; Linn, J.G. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann. Ophthalmol. 1985, 17, 190–192.
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050.
- Troiano, P.; Monaco, G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: A cross-over study. Cornea 2008, 27, 1126–1130.
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213.
- Berkó, S.; Maroda, M.; Bodnár, M.; Eros, G.; Hartmann, P.; Szentner, K.; Szabó-Révész, P.; Kemény, L.; Borbély, J.; Csányi, E. Advantages of cross-linked versus linear hyaluronic acid for semisolid skin delivery systems. Eur. Polym. J. 2013, 49, 2511–2517.
- Barbucci, R.; Leone, G.; Chiumiento, A.; Di Cocco, M.E.; D’Orazio, G.; Gianferri, R.; Delfini, M. Low- and high-resolution nuclear magnetic resonance (NMR) characterization of hyaluronan-based native and sulfated hydrogels. Carbohydr. Res. 2006, 341, 1848–1858.
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. JDD 2011, 10, 990–1000.
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 1110–1119.
- Teh, B.M.; Shen, Y.; Friedland, P.L.; Atlas, M.D.; Marano, R.J. A review on the use of hyaluronic acid in tympanic membrane wound healing. Expert Opin. Biol. Ther. 2012, 12, 23–36.
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489.
- Wende, F.J.; Xuea, Y.; Nestor, G.; Ohrlund, A.; Sandstrom, C. Relaxation and diffusion of water protons in BDDE cross-linked hyaluronic acid hydrogels investigated by NMR spectroscopy—Comparison with physicochemical properties. Carbohydr. Polym. 2020, 248, 116768–116775.
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics. J. Mater. Sci. Mater. Med. 2020, 31, 1–14.
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800.
- Abbruzzese, L.; Rizzo, L.; Fanelli, G.; Tedeschi, A.; Scatena, A.; Goretti, C.; Macchiarini, S.; Piaggesi, A. Effectiveness and safety of a novel gel dressing in the management of neuropathic leg ulcers in diabetic patients: A prospective double-blind randomized trial. Int. J. Low. Extrem. Wounds 2009, 8, 134–140.
- Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Salatin, S.; Saadat, Y.R.; Vahed, S.Z.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536.
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macr. 2019, 140, 245–254.
- Uccello-Barretta, G.; Balzano, F.; Vanni, L.; Sanso, M. Mucoadhesive properties of tamarind-seed polysaccharide/hyaluronic acid mixtures: A nuclear magnetic resonance spectroscopy investigation. Carbohydr. Polym. 2013, 91, 568–572.
- Uccello-Barretta, G.; Nazzi, S.; Zambito, Y.; Di Colo, G.; Balzano, F.; Sanso, M. Synergistic interaction between TS-polysaccharide and hyaluronic acid: Implications in the formulation of eye drops. Int. J. Pharm. 2010, 395, 122–131.
- Silvani, L.; Bedei, A.; De Grazia, G.; Remiddi, S. Arabinogalactan and hyaluronic acid in ophthalmic solution: Experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study). Exp. Eye Res. 2020, 196, 108058–108066.
- D’Adamo, P. Larch Arabinogalactan is a Novel Immune Modulator. J. Naturop. Med. 1996, 4, 32–34.
- Kelly, G.S. Larch arabinogalactan: Clinical relevance of a novel immune-enhancing polysaccharide. Altern. Med. Rev. A J. Clin. Ther. 1999, 4, 96–103.
- Vince, A.J.; McNeil, N.I.; Wager, J.D.; Wrong, O.M. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br. J. Nutr. 1990, 63, 17–26.
- Marzorati, M.; Verhelst, A.; Luta, G.; Sinnott, R.; Verstraete, W.; Van de Wiele, T.; Possemiers, S. In vitro modulation of the human gastrointestinal microbial community by plant-derived polysaccharide-rich dietary supplements. Int. J. Food Microbiol. 2010, 139, 168–176.
- Zippel, J.; Deters, A.; Hensel, A. Arabinogalactans from Mimosa tenuiflora (Willd.) Poiret bark as active principles for wound-healing properties: Specific enhancement of dermal fibroblast activity and minor influence on HaCaT keratinocytes. J. Ethnopharmacol. 2009, 124, 391–396.
- Villarreal, M.L.; Nicasio, P.; Alonso-Cortés, D. Effects of Mimosa tenuiflora bark extracts on WI38 and KB human cells in culture. Arch. Investig. Med. 1991, 22, 163–169.
- Rivera-Arce, E.; Chavez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Gusmann, A.; Lozoya, X. Therapeutic effectiveness of a Mimosa tenuiflora cortex extract in venous leg ulceration treatment. J. Ethnopharmacol. 2007, 109, 523–528.
- Rivera-Arce, E.; Gattuso, M.; Alvarado, R.; Zárate, E.; Agüero, J.; Feria, I.A.; Lozoya, X. Pharmacognostical studies of the plant drug Mimosae tenuiflorae cortex. J. Ethnopharmacol. 2007, 113, 400–408.
- Heinrich, M.; Kuhnt, M.; Wright, C.W.; Rimpler, H.; Phillipson, J.D.; Schandelmaier, A.; Warhurst, D.C. Parasitological and microbiological evaluation of Mixe Indian medicinal plants (Mexico). J. Ethnopharmacol. 1992, 36, 81–85.
- FDA. Larch arabinogalactan. Altern. Med. Rev. 2000, 5, 463.
- De Ferra, L.; Massa, A.; Di Mola, A.; Diehl, B. An effective method for the determination of the enantio-purity of L-α-glycerophosphocholine (L-α-GPC). J. Pharm. Biomed. Anal. 2020, 183, 113152–113157.
- Easteal, A.J.; Edge, V.J.; Woolf, L.A. Isotope Effects in Water. Tracer Diffusion Coefficients for H2180 in Ordinary Water. J. Phys. Chem. 1984, 88, 6060–6063.
- For the Correlation between Diffusion and Self-Diffusion Coefficient See: IUPAC Compendium of Chemical Terminology, 2nd ed.; International Union of Pure and Applied Chemistry: Zurich, Switzerland, 2014.
- Li, D.; Kagan, G.; Hopson, R.; Paul, G.; Williard, P.G. Formula Weight Prediction by Internal Reference Diffusion-Ordered NMR Spectroscopy (DOSY). J. Am. Chem. Soc. 2009, 131, 5627–5634.
- Karsten, W.E.; Lai, C.-J.; Cook, P.F. Inverse Solvent Isotope Effects in the NAD-Malic Enzyme Reaction Are the Result of the Viscosity Difference between D2O and H2O: Implications for Solvent Isotope Effect Studies. J. Am. Chem. Soc. 1995, 117, 5914–5918.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
03 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




