Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seong An | + 2705 word(s) | 2705 | 2021-10-13 07:58:50 | | | |
| 2 | Amina Yu | -3 word(s) | 2702 | 2021-12-03 07:37:03 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2702 | 2022-03-28 03:14:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
An, S. Apiaceae Family Spices in Ameliorating Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/16709 (accessed on 28 February 2026).
An S. Apiaceae Family Spices in Ameliorating Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/16709. Accessed February 28, 2026.
An, Seong. "Apiaceae Family Spices in Ameliorating Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/16709 (accessed February 28, 2026).
An, S. (2021, December 03). Apiaceae Family Spices in Ameliorating Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/16709
An, Seong. "Apiaceae Family Spices in Ameliorating Alzheimer’s Disease." Encyclopedia. Web. 03 December, 2021.
Copy Citation
Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative diseases worldwide. In an effort to search for new strategies for treating AD, natural products have become candidates of choice. Plants are a rich source of bioactive and effective compounds used in treating numerous diseases. Various plant extracts are known to display neuroprotective activities by targeting different pathophysiological pathways in association with the diseases, such as inhibiting enzymes responsible for degrading neurotransmitters, reducing oxidative stress, neuroprotection, inhibiting amyloid plaque formation, and replenishing mitochondrial function.
Alzheimer’s disease
amyloid-beta
antioxidant
enzyme inhibitors
essential oils
natural products
neuroprotective
spices
1. Traditional Spices and Their Neuroprotective Effect
The term “spice” is derived from the Latin word “Species aromatacea”, meaning aromatic species. Spices are dried, aromatic, or pungent edible plant parts (fruit, leaves, seed, root, bark, flower), whose primary purpose in food is seasoning rather than nutrition. The bioactive compounds present in spices such as alkaloids, phenols, terpenes, and flavonoids are responsible for their therapeutic potential. Family Apiaceae (also known as Umbelliferae) has mostly aromatic flowering plants. Numerous species of this family are reported to be rich in essential and vegetable oils and hence are used in the pharmaceutical, cosmetic, perfume, and food industries [1][2]. Reports suggest the role of terpenoids and phenylpropanoids in inhibiting acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity to a variable extent [3], which makes it important that cholinesterase inhibition by essential oils is elucidated more precisely with other chemical components. The vegetable oil obtained from umbelliferous seeds is a very rich source of a rare fatty acid, petroselinic acid (an isomer of oleic acid), and is used in chemical industries [4]. It has already been reported that fatty acids can cross the blood–brain barrier (BBB) via simple diffusion. Additionally, many transport proteins such as fatty acid binding protein 5 (FABP-5), fatty acid transport proteins-1 (FATP-1), FATP-4, and fatty acid translocase (CD36) also assist in the transport. In AD, decreased transport of many fatty acids (linoleic acid, myristic acid, palmitic acid, etc.) has been reported [5]. Moreover, expression of CD36, which is also a microglial receptor involved in the removal of Aβ, is downregulated in AD [6]. The neuroprotective mechanism of the spice active constituents and extracts belonging to the Apiaceae family is summarized in Table 1 and Table 2. The structures of important bioactive compounds from this family (Figure 1) and a diagrammatic representation for the multitarget approach by the spice extracts and phytocompounds are also depicted (Figure 2). The mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease are described below.
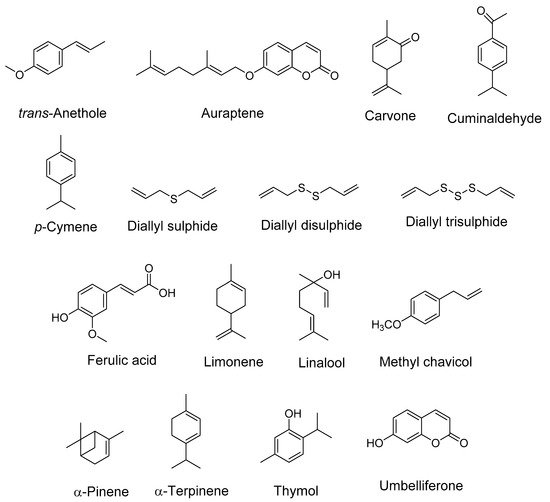
Figure 1. Structures of some important bioactive compounds present in spices (Apiaceae family).

Figure 2. Neuroprotective mechanisms in Alzheimer’s disease displayed by spice extracts and/or natural products belonging to the Apiaceae family.
Table 1. List of biologically active compounds identified from the Apiaceae species.
| Natural Product | Identification in Apiaceae Species | Reported Mechanism Associated with Alzheimer’s Disease | Reference |
|---|---|---|---|
| trans-Anethole | Foeniculum vulgare | AChE-inhibitory activity BChE Inhibitory activity |
[7] |
| Auraptene | Ferula sp. | AChE-inhibitory activity | [8] |
| Carvone | Carum carvi | Neuroinflammatory effects by inhibiting leukotrienes and prostaglandins and modulation of NF-ΚB signaling pathway AChE-inhibitory activity |
[9][10] |
| Cuminaldehyde | Cuminum cyminum | Spatial learning and memory enhancement Modulation of BDNF, Icam, ApoE, and IL-6 genes |
[11] |
| p-Cymene | Coriandrum sativum Trachyspermum ammi Cuminum cyminum |
Improved learning and memory functions Reduced the deposition of amyloid plaques |
[12] |
| Diallyl sulfide Diallyl disulfide Diallyl trisulfide |
Ferula asafoetida | Antioxidant activity by trapping trichloromethyl and trichloromethyl peroxyl free radicals Inhibition of CCl4-induced lipid peroxidation by diallyl disulfide |
[13] |
| Ferulic acid | Ferula asafoetida | AChE-inhibitory activity In vivo cognitive improvement by inhibiting BACE-1, decreasing cleavage of C-terminal APP fragment, neuroinflammatory activity, and stabilization of oxidative stress Enhancement of learning and memory deficits by inhibiting Aβ plaques in vivo Inhibition of Aβ fibrillization and aggregation |
[14][15][16][17][18] |
| Limonene | Anethus graveolens Carum carvi Foeniculum vulgare |
Increased the levels of oxidative markers MDA, SOD, and GSH AChE-inhibitory activity BChE inhibitory activity Suppression of Aβ42-induced cell neurotoxicity Reduction in ROS levels Downregulation of the neurotransmitter Kv3.4 expression Reduction of kinase phosphorylation Neuroinflammatory effects |
[19][20][21][22] |
| Linalool | Coriandrum sativum Carum carvi |
Reduction of lipid peroxidation Cognitive enhancement Antiapoptosis in Aβ42-treated rats |
[23] |
| Methyl chavicol | Foeniculum vulgare | AChE-inhibitory activity | [24] |
| α-Pinene | Trachyspermum ammi Coriandrum sativum Carum carvi |
Improved learning and memory functions by inhibiting AChE and oxidative stressors | [10] |
| α-Terpinene | Carum carvi Coriander sativum Cuminum cyminum Trachyspermum ammi |
AChE-inhibitory activity Inhibition of enzymes responsible for neuronal plasticity and hydrolysis of ADP and ATP |
[10] |
| Thymol | Trachyspermum ammi | Antioxidant activity Inhibition of Aβ plaques in cognitive-impaired rats Neuroinflammatory effects by reduction of the activated astrocytes and microglia and downregulation of COX-2 and iNOS expression in vivo |
[25][26] |
| Umbelliferone | Ferula asafoetida | AChE-inhibitory activity Increased the expression of Nrf2 and heme oxygenase-1 (HO-1) in vivo |
[27] |
Table 2. Neuroprotective activities in Alzheimer’s disease displayed by Apiaceae family spice extracts.
| Plant Species | Extract | Reported Activity Associated with Alzheimer’s Disease | Reference |
|---|---|---|---|
| Anethum graveolens | Methanolic seed extract | Neuroprotective effects in Aβ-induced PC12 cells | [28] |
| Ethanolic leaf extract | Enhancement of learning and memory in vivo AChE-inhibitory activity Amelioration of antioxidant SOD enzyme Reduction of lipid peroxidation |
[29] | |
| Aqueous extract | Improvement of memory impairment in vivo by reducing oxidative stress In vivo lowering of serum cholesterol, inhibition of Aβ deposition, and normalization hippocampal morphology |
[30][31][32] | |
| PM52 extract (combined extract of A. graveolens and Cissampelos pareira) | In vivo Cognitive enhancement by suppressing AChE and reducing levels of ROS | [33] | |
| Carum carvi | Aqueous seed extract | Neuroinflammatory activity by regulating the NF-kB signaling pathway In vivo antioxidant (reduction of lipid peroxidation), adaptogenic, and memory enhancement activities |
[34][35] |
| Essential oil | Inhibition of AChE activity | [36] | |
| Coriandrum sativum | Seed extract | Improvement of memory impairment by increasing the level of mRNA NF-L and decreasing the mRNA nNOS | [37] |
| Cuminum cyminum | Aqueous extract | In vivo memory enhancement, antioxidant activity, and inhibition of AChE | [38][39][40][41] |
| Hexane extract and cumin essential oil | Inhibition of α-synuclein aggregation in PC12 cells | [42] | |
| Foeniculum vulgare | Aqueous extract | In vivo inhibition of lipid peroxidation and antioxidant activity | [43] |
| Ethanolic extract | In vivo neuroprotective effects in lead-induced neurotoxicity by decreasing the levels of oxidative stress and APP isoforms | [44] | |
| Trachyspermum ammi | Seed extract | In vivo learning and memory enhancement by reducing the brain AChE activity, and prevention of oxidative damage by decreasing the levels of MDA and nitrite and increasing GSH | [45] |
| Essential oil | AChE-inhibitory activity In vivo increased in brain antioxidant capacity |
[46] |
The spice extracts and their phytochemicals exert a multitarget approach to ameliorate symptoms of AD (Figure 2). Some components prevent amyloid-beta aggregation by inhibiting the cleavage of the amyloid precursor protein (APP) by β-secretase (BACE-I) (Figure 2A). This causes a shift in the nonamyloidogenic pathway and reduces the levels of Aβ produced [7][36]. Aβ can self-aggregate to form oligomers and eventually amyloid plaques (Figure 2B). Some bioactive components are able to inhibit the formation of amyloid plaques by binding to Aβ, inhibiting aggregation, and thereby promoting the formation of nontoxic oligomers [28][44]. Toxic Aβ monomers and oligomers have been shown to induce microglial activation and proliferation (Figure 2C). Activated microglia secrete proinflammatory cytokines such as IL-1β and IL-6. Some natural products have been shown to reduce the levels of these cytokines [20][22][37]. Microglia also play a role in generating reactive nitrogen species (RNS), which further contribute to neurodegeneration [25][26] (Figure 2D). ROS and RNS irreversibly oxidize DNA and are important mediators of Aβ-induced neuronal cell death in the development of AD (Figure 2E). Many phytochemicals reduce oxidative stress by increasing the levels of antioxidant enzymes and reducing lipid peroxidation [13][19][20][21][22]. Acetylcholine (ACh), a neurotransmitter essential for processing memory and learning, is decreased in both concentration and function in AD (Figure 2F). Decreased levels of ACh can be restored by anticholinesterase activity of various bioactive compounds [7][8][27][46].
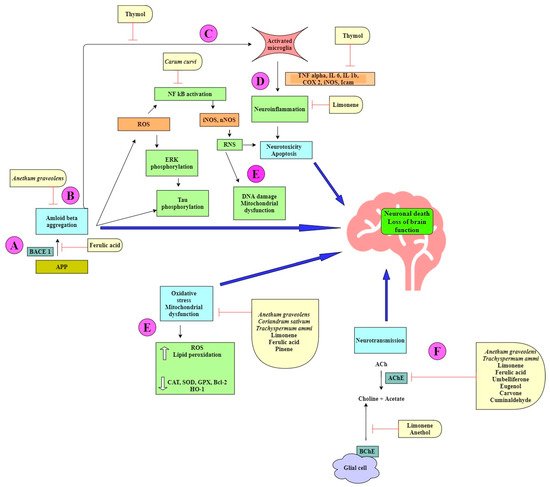
Figure 2. Neuroprotective mechanisms in Alzheimer’s disease displayed by spice extracts and/or natural products belonging to the Apiaceae family.
2. Anethum graveolens
Anethum graveolens (dill) seeds are generally used as a spice, flavoring, and seasoning agent in food such as pickles, salads, etc. Dill essence is rich in flavonoids, a subclass of phytoestrogens that may be accountable for having positive effects on memory enhancement, increasing levels of acetylcholine (Mesripour et al. 2016), and displaying potent antioxidant activity [47]. The most abundant constituents of essential oils in seeds were found to be carvotanacetone (21.76 ± 1.62%), dill apiole (18.65 ± 1.89%), limonene (9.01 ± 1.11%), dill ether (9.13 ± 1.12%), 4-isopropyltoluene (8.24 ± 0.89%), and myrcene (7.44 ± 0.68%) [47].
The methanolic extract of A. graveolens seeds demonstrated moderate neuroprotective effects in PC12 cells treated with Aβ (25–35) aggregates with an ED50 value of 18.8 µg/mL [28]. The administration of A. graveolens ethanolic leaves extract significantly improved the learning and memory damage induced by scopolamine in Morris water maze and elevated plus maze experiments. A substantial decrease in AChE activity, increased activity of brain antioxidant enzymes such as superoxide dismutase, and decreased lipid peroxidation were also observed. The most potent action was seen at a dose of 400 mg/Kg body weight [29].
The memory-enhancing activity of A. graveolens aqueous extract (100, 200, and 300 mg/Kg body weight) was also appraised by the conditioned avoidance response (CAR) technique in rats using Cook’s pole climbing apparatus [31]. Changes in cognition, retention, and recovery in rats were dose dependent. The extract also inhibited lipid peroxidation in both liver and brain tissues, suggesting the role of extract in reducing oxidative stress. In another study, the protective effect of A. graveolens aqueous extract was studied on hypercholesterolemia-induced cognitive deficits (HCDs) and oxidative stress in hippocampus tissues of rats [30][32]. HCD considerably augmented serum cholesterol levels, induced Aβ deposition, transformed morphology of hippocampus, and impaired memory function. However, the changes were reversed by administration of A. graveolens extract, which acted by increasing antioxidant levels in the brain, lowering serum cholesterol, retarding Aβ deposition, and normalizing hippocampal morphology.
PM52, a combined extract of Cissampelos pareira and A. graveolens, was evaluated against age-related cognitive impairment in a rat model. The data proposed that the cognitive-enhancing effect of PM52 might be due to suppression of AchE, resulting in increased levels of acetylcholine, a neurotransmitter that plays an important role in learning and memory and enriching neuron density in hippocampus by reducing the oxidative stress [33].
Hence, A. graveolens extracts and active constituents improved cognitive function in AD brain mainly by inhibiting AChE, improving oxidative stress conditions, and retarding amyloid β aggregation.
3. Carum carvi
Carum carvi (caraway) is a biennial herb, the dried fruit of which is used as a spice due to its pleasing odor and sharp taste. The main components of essential oil are carvone (44.5–95.9%) and limonene (1.5–51.3%) and minor amounts of β-myrcene, trans-dihydrocarvone, trans-carveole (0–0.2%), α-pinene, sabinene, n-octanal, trans-β-ocimene, δ-terpinene, linalool, cis- and trans-limonene oxide, cis-dihydrocarvone, cis-carveol, perillaldehyde, trans-anethole, and trans-β-caryophyllene [48].
Microglia plays a dual role (neuroprotective or neurotoxic) in the progression of AD [49]. Microglia are regarded as initiators of neuroinflammation [50] and might play a role in the atypical networking in AD brain [51]. Various proinflammatory and neurotoxic substances, such as NO, iNOS, and COX-2, are produced by activated microglia. Therefore, reducing neuroinflammation by reducing microglia activation could be a promising therapeutic target in treatment of neuroinflammatory-mediated neurodegenerative diseases such as AD. It has already been reported that natural products with antineuroinflammatory activity are able to produce antiamyloid [52]. In an in vitro study, C. carvi aqueous extract was evaluated for its protective effects on LPS-activated neuroinflammation in BV-2 microglial cells. The extract inhibited LPS-induced phosphorylation/degradation of IκBα and translocation of NF-κB/p65 subunit in a concentration-dependent manner, which means the C. carvi extract plays an important role in regulating NF-κB signaling [34]. Carvone, the major component of C. carvi oil, is known to exhibit anti-inflammatory properties by inhibiting the synthesis of leukotrienes and prostaglandins [9]. Therefore, it is quite possible that carvone plays a role in modulation of the NF-κB pathway. Antioxidant, adaptogenic, and memory-enhancer activities of C. carvi aqueous extract were evaluated in rats using Cook’s pole climbing apparatus. The extract decreased lipid peroxidation in liver and brain homogenates [35]. Essential oil (EO) of C. carvi displayed strong in vitro anti-AChE activity (IC50 = 0.82 ± 0.05 mg/mL) compared to the reference drug galantamine (IC50 = 1.05 ± 0.05 mg/mL) [36]. Pharmacokinetics profiling of selected components of essential oils indicated their ability to penetrate the blood–brain barrier moderately (log BB values = 0.818–0.478). They also demonstrated high CaCO-2 permeability (log Papp values > 0.90 cm/s). All tested compounds were predicted neither substrates nor inhibitors of the human cytochrome P450 (CYP) isoforms. The boiled-egg graph (WLOGP vs. TPSA) prediction of GI absorption and BBB permeation, which helps in the calculation of polarity and lipophilicity, indicated that they possess a high probability of brain penetration [36].
Carvone, the main constituent of C. carvi, has been reported as an AChE inhibitor (IC50 = 2.9 ± 0.12 mM) compared to the reference drug galantamine (IC50 = 0.14 ± 0.005 mM) [10]. The inhibition is noncompetitive [53]. Additionally, docking studies revealed a putative H-bond interaction between the carvone and Tyr337 (2.92Å) of AChE, creating an anionic subsite. Other binding sites include Trp86, Tyr133, Tyr337, Phe338 (an anionic subsite), His447, Ser203 (an esteratic site), Gly121, Gly122 (oxyanion hole), Ile451, Gly448, Glu202, Gly120, and Ser125, all of which are most important portions of the AchE binding site [10]. In a recent study, (‒)-cis-carveol, a reduction product of carvone, improved Aβ1-42-induced memory deficits in an animal model, examined by Y-maze and radial arm maze in vivo tests [54]. The biochemical analyses of the hippocampus homogenates showed a reduction in oxidative stress parameters caused by Aβ1-42, suggesting the role of carveol in neuroprotection.
The second most important constituent in C. carvi is limonene, a monoterpene. Its protective role in spatial memory and anxiety has been established in a rat model exposed to immobilized stress [55]. Limonene also had a positive effect on scopolamine-induced amnesia, where it improved the modifications caused by scopolamine in a short-term memory test. It ameliorated the levels of oxidative stress markers (MDA, SOD, GSH) and inhibited AchE and BchE [3][20]. A study examined the effectiveness of limonene against Aβ1-42-induced neurotoxicity in a Drosophila model of AD. The results showed that limonene suppressed the neuronal cell death induced by Aβ42 and reduced oxidative stress, which prevented ERK phosphorylation [22]. In another study, the neuroprotective effect of limonene against neurotoxicity elicited by Aβ1-42 in Hoechst 33,258 cell lines was observed. Limonene decreased ROS production and prevented the upregulation of Kv3.4 (voltage-gated potassium channel) activity at 10 µg/mL. This channel is overexpressed in AD and other neurodegenerative diseases [19]. Downregulation of Kv3.4 expression by limonene prevented cell death in primary cortical neurons, thus confirming its neuroprotective function in AD [21]. Moreover, limonene displayed a specific activity almost comparable to galantamine, the reference drug used against AchE.
Hence, maintaining the levels of antioxidant enzymes, inhibiting AchE, suppressing neuroinflammation and downregulating voltage-gated potassium channels are the main roles of C. carvi extract and active components in combating AD.
4. Coriandrum sativum
Coriandrum (coriander) is a feathery annual plant, used as both an herb and a spice. The main constituents of coriander oil are linalool (64.2−79.9%), γ-terpinene (5.8−13.6%), neryl acetate (2.3−8.4%), α-pinene (2.8−7.1%), and p-cymene (1.1−3.6%) [56]. In the Aβ1-42 AD model, a test group of rats as made to inhale essential oil (1% and 3%) from C. sativum seed. Inhalation of essential oil considerably reduced levels of LDH and MDA, with an increase in glutathione peroxidase levels in the hippocampal region of rats. Additionally, there were fewer amyloid deposits in rats treated with EO. Specifically, linalool was found to be the active constituent in the EO; therefore, it can be speculated that linalool is responsible for cognitive-enhancing effects, along with antiapoptotic activities in Aβ1-42-treated rats. The antioxidant defense, along with a decrease in lipid peroxidation, could be correlated with involvement of linalool in neuroprotection [23]. C. sativum seed extract is reported to be nontoxic in up to 3000 mg/kg body weight (BW) and, thus, can be considered safe for intake [57]. In a recent experiment, C. sativum seed extract (200 mg/Kg BW) improved memory impairment in senescence-accelerated mouse-prone 8 (SAMP8) mice. The mRNA levels of nNOS were higher in diseased the frontal lobe of diseased animals, which decreased significantly with the treatment of extract, suggesting that C. sativum can reduce the production of RNS and ROS and thus improve oxidative stress conditions. Moreover, the mRNA level of neurofilament light (NF-L), an important protein in memory retention and synaptic plasticity, was found lower in the frontal lobe and hippocampus of untreated mice, indicating neuronal damage. However, the mRNA levels NF-L were elevated after extract administration [37], indicating the role of C. sativum in neuroprotection. Previous studies have shown that α-pinene, γ-terpinene, and many monoterpenoids have anti-AchE activity [10][58][59]. As these phytocompounds are present in C. sativum, it is very much likely that its extract will also show anti-AchE activity.
Therefore, as described above, C. sativum extract and its bioactive compounds play an imperative role in neuroprotection by reducing ROS/RNS, elevating the level of an important protein involved in synaptic plasticity, and suppressing AchE activity.
References
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Chokr, A.; Kanaan, H.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crop. Prod. 2018, 111, 92–98.
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671.
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and phenylpropanoids as acetyl- and butyrylcholinesterase inhibitors: A comparative study. Curr. Alzheimer Res. 2019, 16, 963–973.
- Nguyen, Q.H.; Talou, T.; Evon, P.; Cerny, M.; Merah, O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020, 326, 127034.
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746.
- Dobri, A.M.; Dudău, M.; Enciu, A.M.; Hinescu, M.E. CD36 in Alzheimer’s disease: An overview of molecular mechanisms and therapeutic targeting. Neuroscience 2020, 453, 301–311.
- Bhadra, S.; Mukherjee, P.K.; Kumar, N.S.; Bandyopadhyay, A. Anticholinesterase activity of standardized extract of Illicium verum Hook f fruits. Fitoterapia 2011, 82, 342–346.
- Epifano, F.; Molinaro, G.; Genovese, S.; Ngomba, R.T.; Nicoletti, F.; Curini, M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci. Lett. 2008, 443, 57–60.
- Agrahari, P.; Singh, D.K. A review on the pharmacological aspects of Carum carvi. J. Biol. Earth Sci. 2014, 4, M1–M13.
- Wojtunik-Kulesza, K.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343.
- Omari, Z.; Kazunori, S.; Sabti, M.; Bejaoui, M.; Hafidi, A.; Gadhi, C.; Isoda, H. Dietary administration of cumin-derived cuminaldehyde induce neuroprotective and learning and memory enhancement effects to aging mice. Aging 2021, 13, 1671–1685.
- Nahavandi, B.S.; Yaghmaei, P.; Ahmadian, S.; Ebrahim-Habibi, A.; Ghobeh, M. Effects of terpinolene and physical activity on memory and learning in a model of Alzheimer’s disease among rats. Qom Univ. Med. Sci. J. 2020, 14, 25–33.
- Fanelli, S.L.; Castro, G.D.; de Toranzo, E.G.; Castro, J.A. Mechanisms of the preventive properties of some garlic components in the carbon tetrachloride-promoted oxidative stress. Diallyl sulfide, diallyl disulfide, allyl mercaptan and allyl methyl sulfide. Res. Commun. Mol. Pathol. Pharmacol. 1998, 102, 163–174.
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long term administration of ferulic acid on microglial activation induced by intercerebroventricular injection of beta amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121.
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774.
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782.
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758.
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96.
- Angulo, E.; Noé, V.; Casadó, V.; Mallol, J.; Gomez-Isla, T.; Lluis, C.; Ferrer, I.; Ciudad, C.J.; Franco, R. Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer’s disease. J. Neurochem. 2004, 91, 547–557.
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Orhan, I.E.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519.
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by Aβ1-42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937.
- Tramutola, A.; Triani, F.; Di Domenico, F.; Barone, E.; Cai, J.; Klein, J.B.; Perluigi, M.; Butterfield, D.A. Poly-ubiquitin profile in Alzheimer disease brain. Neurobiol. Dis. 2018, 118, 129–141.
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β (1–42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202.
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Naturforsch. C 2016, 71, 393–402.
- Asadbegi, M.; Komaki, A.; Salehi, I.; Yaghmaei, P.; Ebrahim-Habibi, A.; Shahidi, S.; Sarihi, A.; Asl, S.S.; Golipoor, Z. Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res. Bull. 2018, 137, 338–350.
- Azizi, Z.; Ebrahimi, S.; Saadatfar, E.; Kamalinejad, M.; Majlessi, N. Cognitive enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 2012, 23, 241–249.
- Hindam, M.O.; Sayed, R.H.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a strep-tozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytother. Res. 2020, 34, 2351–2365.
- Park, S.Y.; Kim, H.S.; Hong, S.S.; Sul, D.; Hwang, K.W.; Lee, D. The neuroprotective effects of traditional oriental herbal medicines against β-amyloid-induced toxicity. Pharm. Biol. 2009, 47, 976–981.
- Kumar, N.; Dhiman, C.; Kothiyal, P. Evaluation of Anethum graveolens extract on memory impaired mice. Indo Am. J. Pharm. Sci. 2017, 4, 1965–1975.
- Heshami, N.; Mohammadali, S.; Komaki, A.; Tayebinia, H.; Karimi, J.; Oshaghi, E.A.; Hashemnia, M.; Khodadadi, I. Favorable effects of dill tablets and Ocimum basilicum L. extract on learning, memory, and hippocampal fatty acid composition in hypercholesterolemic rats. Iran. J. Basic Med. Sci. 2021, 24, 300–311.
- Koppula, S.; Choi, D.K. Anethum graveolens Linn (Umbelliferae) extract attenuates stress-induced urinary biochemical changes and improves cognition in scopolamine induced amnesic rats. Trop. J. Pharm. Res. 2011, 10, 47–54.
- Mohammadali, S.; Heshami, N.; Komaki, A.; Tayebinia, H.; Oshaghi, E.A.; Karimi, J.; Hashemnia, M.; Khodadadi, I. Dill tablet and Ocimum basilicum aqueous extract: Promising therapeutic agents for improving cognitive deficit in hypercholesterolemic rats. J. Food Biochem. 2020, 44, e13485.
- Thukham-Mee, W.; Wattanathorn, J. Evaluation of safety and protective effect of combined extract of Cissampelos pareira and Anethum graveolens (PM52) against age-related cognitive impairment. Evid.-Based Complement. Altern. Med. 2012, 2012, 674101.
- Kopalli, S.R.; Koppula, S. Carum carvi Linn (Umbelliferae) attenuates lipopolysaccharide-induced neuroinflammatory responses via regulation of NF-κB signaling in BV-2 Microglia. Trop. J. Pharm. Res. 2015, 14, 1041–1047.
- Koppula, S.; Kopalli, S.R.; Sreemantula, S. Adaptogenic and nootropic activities of aqueous extracts of Carum carvi Linn (Caraway) fruit: An experimental study in Wistar rats. Aust. J. Med. Herb. 2009, 21, 72–78.
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 2021, 26, 3625.
- Mima, Y.; Izumo, N.; Chen, J.-R.; Yang, S.-C.; Furukawa, M.; Watanabe, Y. Effects of Coriandrum sativum seed extract on aging-induced memory impairment in Samp8 mice. Nutrients 2020, 12, 455.
- Kim, J.B.; Kopalli, S.R.; Koppula, S. Cuminum cyminum Linn (Apiaceae) extract attenuates MPTP-induced oxidative stress and behavioral impairments in mouse model of Parkinson’s disease. Trop. J. Pharm. Res. 2016, 15, 765–772.
- Koppula, S.; Choi, D.K. Cuminum cyminum extract attenuates scopolamine-induced memory loss and stress-induced urinary biochemical changes in rats: A non-invasive biochemical approach. Pharm. Biol. 2011, 49, 702–708.
- Kumar, S.; Brijeshlata, D.S.; Dixit, S. Screening of traditional Indian spices for inhibitory activity of acetylcholinesterase and butyrylcholinesterase enzymes. Int. J. Pharm. Bio. Sci. 2012, 3, 59–65.
- Fang, L.; Wang, X.; Guo, L.; Liu, Q. Antioxidant, antimicrobial properties and chemical composition of cumin essential oils extracted by three methods. Open Chem. 2018, 16, 291–297.
- Morshedi, D.; Aliakbari, F.; Tayaranian-Marvian, A.; Fassihi, A.; Pan-Montojo, F.; Pérez-Sánchez, H. Cuminaldehyde as the major component Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food Sci. 2015, 80, H2336–H2345.
- Koppula, S.; Kumar, H. Foeniculum vulgare Mill (Umbelliferae) attenuates stress and improves memory in Wister rats. Trop. J. Pharm. Res. 2013, 12, 553–558.
- Bhatti, S.; Ali Shah, S.A.; Ahmed, T.; Zahid, S. Neuroprotective effects of Foeniculum vulgare seeds extract on lead-induced neurotoxicity in mice brain. Drug Chem. Toxicol. 2018, 41, 399–407.
- Soni, K.; Parle, M. Trachyspermum ammi seeds supplementation helps reverse scopolamine, alprazolam and electroshock induced amnesia. Neurochem. Res. 2017, 42, 1333–1344.
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris essential oil protects zebrafish against cognitive dysfunction by regulating cholinergic and antioxidants systems. Antioxidants 2020, 9, 1083.
- Ozliman, S.; Yaldiz, G.; Camlica, M.; Ozsoy, N. Chemical components of essential oils and biological activities of the aqueous extract of Anethum graveolens L. grown under inorganic and organic conditions. Chem. Biol. Technol. Agric. 2021, 8, 20.
- Raal, A.; Arak, E.; Orav, A. The content and composition of the essential oil found in Carum carvi L. commercial fruits obtained from different countries. J. Essent. Oil Res. 2012, 24, 53–59.
- Onuska, K.M. The dual role of microglia in the progression of Alzheimer’s disease. J. Neurosci. 2020, 40, 1608–1610.
- Filipov, N.M. Overview of peripheral and central inflammatory responses and their contribution to neurotoxicity. Adv. Neurotoxicol. 2019, 3, 169–193.
- Passamonti, L.; Tsvetanov, K.A.; Jones, P.S.; Bevan-Jones, W.R.; Arnold, R.; Borchert, R.J.; Mak, E.; Su, L.; O’Brien, J.; Rowe, J. Neuroinflammation and functional connectivity in Alzheimer’s disease: Interactive influences on cognitive performance. J. Neurosci. 2019, 39, 7218–7226.
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965.
- López, M.D.; Campoy, F.J.; Pascual-Villalobos, M.J.; Muñoz-Delgado, E.; Vidal, C.J. Acetylcholinesterase activity of electric eel is increased or decreased by selected monoterpenoids and phenylpropanoids in a concentration-dependent manner. Chem. Biol. Interact. 2015, 229, 36–43.
- Hritcu, L.; Boiangiu, R.S.; de Morais, M.C.; de Sousa, D.P. (‒)-cis-Carveol, a natural compound, improves β-amyloid-peptide 1-42-induced memory impairment and oxidative stress in the rat hippocampus. BioMed Res. Int. 2020, 2020, 8082560.
- Bigdeli, Y.; Asle-Rousta, M.; Rahnema, M. Effects of limonene on chronic restraint stress-induced memory impairment and anxiety in male rats. Neurophysiology 2019, 51, 107–113.
- Ebrahimi, S.N.; Hadian, J.; Ranjbar, H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294.
- Patel, D.; Desai, S.; Devkar, R.; Ramachandran, A.V. Acute and sub-chronic toxicological evaluation of hydro-methanolic extract of Coriandrum sativum L. seeds. EXCLI J. 2012, 11, 566–575.
- Picollo, M.I.; Toloza, A.C.; Mougabure, C.G.; Zygadlo, J.; Zerba, E. Anticholinesterase and pediculicidal activities of monoterpenoids. Fitoterapia 2008, 79, 271–278.
- Yu, Z.; Wang, B.; Yang, F.; Sun, Q.; Yang, Z.; Zhu, L. Chemical composition and anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stage. Iran. J. Pharm. Res. 2011, 10, 265–271.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
28 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





