
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md Khadem Ali | + 2088 word(s) | 2088 | 2021-08-31 10:18:18 | | | |
| 2 | Catherine Yang | Meta information modification | 2088 | 2021-12-02 05:09:35 | | |
Video Upload Options
Pulmonary arterial hypertension (PAH) is a debilitating condition of the pulmonary circulatory system that occurs in patients of all ages and if untreated, eventually leads to right heart failure and death.
1. Introduction
Pulmonary arterial hypertension (PAH) is a devastating cardio-pulmonary disease characterized by progressive remodeling of the pulmonary vessels that results in a narrowing of the arterial lumen, leading to an increase in pulmonary vascular resistance and elevation of pulmonary arterial pressure and eventually right ventricular (RV) failure and death. Dysfunction of pulmonary vascular cells such as pulmonary arterial endothelial cells (PAECs), pulmonary arterial smooth muscle cells (PASMCs), fibroblasts, dysregulation of the immune system, and impaired angiogenesis are collaboratively involved in promoting pulmonary vascular remodeling [1]. While PAH is a relatively rare disease, affecting about one to two persons per million yearly, the morbidity and mortality rates are high as it leads to RV failure and death within 2–3 years if left untreated [2][3]. The exact cause for PAH is unknown, but it may be associated with genetic (mutations) or epigenetic changes, immune and inflammatory triggers, environmental factors (e.g., hypoxia, viral infections, anorectic agents etc.), and altered metabolism. Our understanding of the molecular and cellular basis for PAH pathogenesis has significantly progressed. While currently available treatment options have been shown to increase survival and improve quality of life, the disease however remains incurable. Therefore, new effective therapies are urgently needed. Understanding the molecular mechanisms that underlie the pathogenesis of PAH is essential in discovering novel treatments for PAH.
Non-coding RNAs (ncRNAs), mostly micro-RNAs, have recently become an area of increasing interest for the role they play in health and disease. Emerging evidence suggests that a subgroup of ncRNAs, long non-coding RNAs (lncRNAs), endogenously expressed RNAs (≥200 nt length) that lack protein coding ability, nevertheless play important roles in a range of pathophysiological processes, such as regulation of cellular activities (e.g., proliferation, migration, apoptosis, angiogenesis, metabolism), inflammatory and immune responses, and vascular angiogenesis [4][5][6][7][8][9][10]. LncRNAs have also been found to play a key regulative role in osteogenesis [11]. Based on their genomic location near protein-coding regions, lncRNAs are classified into five categories: sense, antisense, intronic, intergenic, and bidirectional [12]. Generally, lncRNAs are transcribed by RNA polymerase II and the premature lncRNAs are post transcriptionally modified, such as 5′- ends capped with 7-methyl-guanosine, alternatively spliced, 3′-polyadenylated similar to protein coding mRNAs [13]. In addition, several other pathways contribute to the generation of lncRNAs. For example, RNA polymerase III generates non-polyadenylated lncRNAs, and some lncRNAs are generated during snoRNA production [14]. Compared with mRNAs, lncRNAs expression is highly cell type- and tissue-specific, and they have longer but fewer exons [15]. LncRNAs are usually of low abundance, less evolutionary conserved, mostly localized in the nucleus but also found in the cytoplasm, while their subcellular localization can influence the regulation mechanism and functional models [16][17]. For a large number of lncRNAs their precise function is still unknown, therefore, their exact classification is unclear because of the lack of detailed functional characterization. LncRNAs regulate gene expression at the epigenetic, transcriptional, and post-transcriptional levels via acting as guide, decoy, scaffold, and signaling molecules [18]. LncRNAs can also indirectly regulate gene expression via acting as competing endogenous RNAs, sponging off miRNAs from their mRNA targets [19]. Previous studies suggest that abnormally expressed lncRNAs are strongly linked with the pathogenesis of many human diseases, including cardiovascular disorders [20][21][22], respiratory disease [23], and different types of cancer [24][25].
Cancer and PAH exhibit many similar characteristics, such as sustained cell proliferation and apoptosis resistance, abnormal angiogenesis, and dysregulated cellular metabolism [26]. LncRNAs may regulate cell proliferation, migration, cell cycle, apoptosis, DNA damage, metabolism, and immune function in human cancers through different mechanisms [14][27]. There is evidence that lncRNAs are also involved in the proliferation, apoptosis, and cell cycle of PAECs and PASMCs in PAH [28]. Recently, lncRNAs have also been shown to be implicated in pulmonary vascular remodeling and PAH pathogenesis [29]. In this review, we discuss lncRNAs and the molecular mechanisms of their involvement in the development and progression of PAH.
2. LncRNAs in PAH
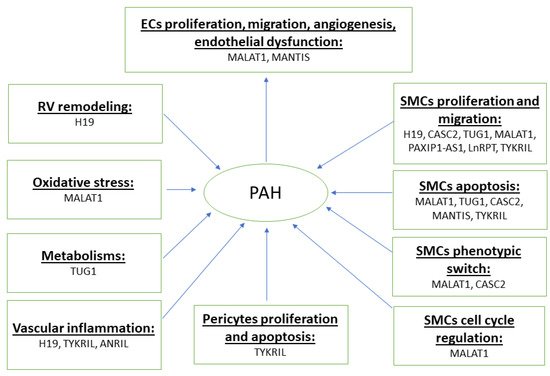
Emerging studies suggest that lncRNAs are differentially expressed in key pulmonary vascular cells such as PAECs and PASMCs, which regulate a range of cellular and biological processes and contribute to pulmonary vascular remodeling and PAH pathogenesis. Herein, we review the roles of lncRNAs in pathological processes in PAH ( Figure 1 and Table 1 ).
| LncRNA | Subcellular Localization | Experimental Models Used | Expression in PAH | Key Findings | Mechanisms | Ref |
|---|---|---|---|---|---|---|
| TYKRIL | Nucleus and cytoplasm | Human PAH PCLS. Hypoxia-induced PH in HPASMCs |
↑ in PASMC, pericytes | Enhances proliferation, inhibits apoptosis of HPASMCs under hypoxia. Regulates pulmonary vascular remodeling in PCLS. |
p53/PDGFRβ axis | [30] |
| H19 | - | MCT-, and PAB-induced PH rat models | ↑ in decompensated RV of PAH patients | Correlates with fibrosis and RV hypertrophy in PAH patients. Compromises the RV functions in PAH, inhibition showed benefits in two PH animal models. Plasma levels of H19 showed biomarker potentials in two independent IPAH cohorts |
EZH2 | [31] |
| H19 | - | MCT-induced PH mouse and rat model | ↑ in serum, lung | Increases PDGF-induced PASMC proliferation. Depletion of H19 protects against MCT-induced PA remodeling and PAH in mice |
miRNA let-7b/ AT1R | [32] |
| MEG3 | Predominantly in cytoplasm | Hypoxia-induced PH in HPASMCs | ↓ in lung, PA, PASMC | Inhibits HPASMCs proliferation and migration | miR-21/PTEN; p53 pathway | [33][34] |
| MEG3 | Predominantly in cytoplasm | Hypoxia-induced PH in mice and HPASMCs | ↑ in PASMC | Downregulation inhibits hypoxia-induced PH development in mice, Hyperactivates cell cycle progression, increases proliferation of PASMC under hypoxia. | miR-328-3p/IGF1R | [35] |
| CASC2 | - | Hypoxia-induced PH in rats and PASMCs | ↓ in PA, PASMC | Inhibits hypoxia-induced vascular remodeling in vivo, inhibits cell proliferation, migration, and phenotypic switch of PASMC | α-SMA | [36] |
| PAXIP1-AS1 | Both nucleus and cytoplasm | Small PAs of IPAH patients | ↑ IPAH-PASMCs and PAs | Inhibition promotes apoptosis and inhibits PASMC proliferation and migration | Paxillin | [37] |
| TUG1 | Both nucleus and cytoplasm | Hypoxia-induced PH in mice and HPASMCs | ↑ in PA | Inhibition prevents and inhibits PH in hypoxia-induced PH mouse models. Promotes HPASMC proliferation and cell cycle progression in vitro |
miR-374c/Foxc1, notch signalling | [38][39] |
| MANTIS | Nucleus | MCT-induced PH rat models | ↓ in lung | Promotes apoptosis, accelerates endothelial angiogenic function. | BRG1 | [40] |
| ANRIL | - | Hypoxia-induced PH in HPASMCs | ↓ in PASMC | Promotes proliferation and migration of PASMC | Unknown | [41] |
| HOXA-AS3 | - | Hypoxia-induced PH in HPASMCs | ↑ in lung, PASMC | Modulates cell cycle and enhance proliferation in PASMC | HOXA3 | [42] |
| MALAT1 | - | - | ↑ in PA, PASMC | Accelerates cell cycle progression as well as pulmonary vascular remodeling | hsa-miR-124-3p.1/KLF5 | [43] |
| MALAT1 | - | Hypoxia-induced PH in HPASMCs | ↑ in plasma and hypoxic HPASMCs | Knockdown reduces HPASMCs proliferation and migration while promotes their apoptosis | miR-503/TLR4 Axis | [44] |
| UCA1 | Predominantly in cytoplasm | Hypoxia-induced PH in HPASMCs | ↑ in PASMC | Promotes proliferation and inhibits apoptosis in PASMC | hnRNP I | [45] |
| SMILR | - | Hypoxia-induced PH in HPASMCs, MCT-induced PH in Rats |
↑ in serum | Regulates vascular remodeling and PAH | RhoA/ROCK/miR-141 signaling | [46] |
| TCONS_00034812 | - | Hypoxia-induced PH in Rats and PASMCs | ↓ in PA, PASMC | Promotes proliferation and inhibits apoptosis of PASMC | Stox1/MAPK signaling | [47] |
| LincRNA-COX2 | - | Hypoxia-induced PH in PASMCs | ↑ in blood, PASMCs | Promotes PASMCs proliferation | LincRNA-COX2/miR-let-7a/STAT3 axis | [48] |
| RPS4L | - | Hypoxia-induced PH in mice and HPASMCs | ↓ in PASMC | Ameliorates hypoxia-induced PH in vivo. Modulates proliferation, migration, and cell cycle progression of PASMC | ILF3/HIF1α | [49] |
| LnRPT | - | PDGF-BB-induced hyperproliferation of rat PASMCs | ↓ in PASMC | Promotes PASMC proliferation | Notch signaling pathway | [50] |
| PAHRF | - | Hypoxia-induced PAH in PASMC | ↓ in PAs of PAH patients and hypoxic PASMC and | Downregulation of PAHRF promotes PASMC proliferation and inhibits apoptosis | PAHR/ miR-23a-3p/MST1 axis | [51] |
| GAS5 | - | Hypoxia-induced PH in rats and PASMC | ↓ in rats PH models and hypoxic PASMC | Downregulation of Gas5 promotes PASMC proliferation | Gas5/miR-23b-3p/KCNK3 axis | [52] |
Using RNAseq data analysis of PASMC and lung pericytes from IPAH patients and hypoxia-exposed PASMC and pericytes, Zehendner et al., identified a novel lncRNA, called TYKRIL which is significantly increased in all four hyperproliferative conditions [30]. Under these hyperproliferative conditions, TYKRIL has been shown to promote proliferation and inhibit apoptosis in PASMC and pericytes by the p53/PDGF signaling axis [30]. Since TYKRIL has poor conservation in animals, the authors performed studies in ex vivo precision-cut lung slices (PCLS) collected from lungs of IPAH patients and demonstrated that GapmeR-mediated knockdown of TYKRIL in PCLS reverses pulmonary vascular remodeling [30], suggesting that TYKRIL has therapeutic potential for PAH treatment.
LncRNA PAHRF, also known as NONHSAT169231.1, is located on chromosome 14 in the human genome. HPASMCs exposed to hypoxia as well as PAs from patients with PAH were found to have low PAHRF expression compared to the corresponding control samples [51]. PAHRF overexpression inhibited proliferation and promoted apoptosis in HPASMCs, whereas knockdown resulted in opposite effects [51]. Further mechanistic studies suggest that downregulation of PAHRF expression serves as a miR-23a-3p sponge to promote pulmonary vascular remodeling in hypoxic pulmonary hypertension by stimulating the proliferation of HPASMCs and inhibiting their apoptosis by downregulating MST1 expression [51]. This study suggests that PAHRF/miR-23a-3p/ MST1 signaling axis could be a possible therapeutic target for PAH treatments by improving pulmonary vascular remodeling.
3. LncRNAs as a Potential Biomarkers in PAH
LncRNAs could be used as a potential biomarkers in PAH. For instance, in a study of 736 healthy controls and 587 Chinese PAH patients, a functional polymorphism of lncRNA MALAT1 (rs619586A >G) has been shown to be linked with a decreased susceptibility to PAH. Mechanistically, this polymorphism in lncRNA MALATI created a miRNA sponge site for miR-214 and thereby increased the expression of X box binding protein 1 (XBP1) expression [53]. As another example, qPCR analysis of plasma levels of lncRNA H19 expressions were shown to be higher in IPAH patients in two cohorts of PAH patients and controls from Canada (52 IPAH and 57 controls) and the UK (75 IPAH and 54 controls). The circulatory H19 levels, partially discriminating PAH patients from controls, were modestly linked with RV function in PAH patients, and were predominantly increased in PAH patients with a low cardiac index. Finally, higher levels of H19 were associated with poorer long-term outcomes in both cohorts. In contrast, a previous study found no difference in the expression of H19 in plasma between eight PAH patients and eight healthy controls, as assessed by qPCR [54]. It is notable that the authors of this study did not specify which PAH types were used (e.g., idiopathic, heritable, or connective tissue diseases associated etc.). In this relatively small sample size analysis, low abundance of H19 in plasma may cause high variability that could potentially render the results statistically insignificant. Further studies are needed to confirm these findings in a larger cohort of patients with different PAH types. Taken together, these findings suggest a potential biomarker role of lncRNAs in PAH.
4. Therapeutic Potential of lncRNAs in PAH
LncRNAs play a critical role in the pathogenesis of PAH; therefore, a deeper understanding of their function and mechanism of action is of utmost importance to develop novel and effective therapies for this disease. Since expression of lncRNAs are highly cell/tissue-specific, they could be used as a potential tools for personalized treatments. Injection of BC-819, a double-stranded DNA plasmid containing a gene for diphtheria toxin under the regulation of the H19 gene promoter into a mouse bladder tumor, for example, decreased tumor size [55]. Combined treatment of BC-819 and Bacilli Calmette Guerin (BCG) was shown to significantly improve patients’ outcomes with a recurrence free bladder cancer survival rate of 54.1% and a progression free survival rate of 75.7% over 24-months in a Phase II clinical trial in patients with non-muscle invasive bladder cancer, suggesting that lncRNA-based treatments have a promising future.
To date, no clinical trials targeting lncRNAs in PAH have been carried out as many obstacles remain. Foremost, lncRNAs are poorly conserved across species, which makes it difficult to develop and test new drugs for clinical translation of human lncRNAs. Also, several lncRNAs have shown promising therapeutic potential in in vitro and in vivo PAH studies, however, in clinical studies the use of lncRNA therapy will need to be handled with caution due to the potential effect on multiple cellular pathways in different cell types and tissues. Antisense oligonucleotides, RNA interference drugs, and CRISPR genome editing tools can be used to alter lncRNA expression as a potential therapeutic option for treating PAH. Furthermore, before a successful translation of preclinical lncRNA studies into clinical use, other issues must be resolved such as improving the stability of RNA-based drugs in the circulation, identifying the secondary structure of lncRNAs, determining the proper route of delivery, improving the efficiency and duration of delivery systems, determining the speed of onset and duration of their action, and limiting off-target effects and adjusting patient-specific doses.
References
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887.
- Benza, R.L.; Miller, D.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An Evaluation of Long-term Survival from Time of Diagnosis in Pulmonary Arterial Hypertension From the REVEAL Registry. Chest 2012, 142, 448–456.
- Peacock, A.J.; Murphy, N.F.; Mcmurray, J.; Caballero, L.; Stewart, S. An epidemiological study of pulmonary arterial hypertension. Eur. Respir. J. 2007, 30, 104–109.
- Liu, X.; Xiao, Z.-D.; Han, L.; Zhang, J.; Lee, S.-W.; Wang, W.; Lee, H.; Zhuang, L.; Chen, J.; Lin, H.-K.; et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016, 18, 431–442.
- Sun, M.; Nie, F.; Wang, Y.; Zhang, Z.; Hou, J.; He, D.; Xie, M.; Xu, L.; De, W.; Wang, Z.; et al. LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT. Cancer Res. 2016, 76, 6299–6310.
- Gasri-Plotnitsky, L.; Ovadia, A.; Shamalov, K.; Nizri-Megnaji, T.; Meir, S.; Zurer, I.; Cohen, C.J.; Ginsberg, D. A novel lncRNA, GASL1, inhibits cell proliferation and restricts E2F1 activity. Oncotarget 2017, 8, 23775–23786.
- Peng, W.X.; Koirala, P.; Mo, Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667.
- Leeper, N.J.; Maegdefessel, L. Non-coding RNAs: Key regulators of smooth muscle cell fate in vascular disease. Cardiovasc. Res. 2018, 114, 611–621.
- Haemmig, S.; Simion, V.; Feinberg, M.W. Long Non-Coding RNAs in Vascular Inflammation. Front. Cardiovasc. Med. 2018, 5, 22.
- Kumar, M.M.; Goyal, R. LncRNA as a Therapeutic Target for Angiogenesis. Curr. Top. Med. Chem. 2017, 17, 1750–1757.
- Lanzillotti, C.; De Mattei, M.; Mazziotta, C.; Taraballi, F.; Rotondo, J.C.; Tognon, M.; Martini, F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 646032.
- Hermans-Beijnsberger, S.; van Bilsen, M.; Schroen, B. Long non-coding RNAs in the failing heart and vasculature. Non-Coding RNA Res. 2018, 3, 118–130.
- Dhanoa, J.K.; Sethi, R.S.; Verma, R.; Arora, J.S.; Mukhopadhyay, C.S. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J. Anim. Sci. Technol. 2018, 60, 1–10.
- Taniue, K.; Akimitsu, N. The Functions and Unique Features of LncRNAs in Cancer Development and Tumorigenesis. Int. J. Mol. Sci. 2021, 22, 632.
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Merkel, A.; Gonzalez, D.; Lagarde, J.; et al. The GENCODE v7 Catalogue of Human Long Non-Coding RNAs: Analysis of Their Structure, Evolution and Expression. Genome Res. 2012, 22, 1775–1789.
- Xiao, J.-H.; Hao, Q.-Y.; Wang, K.; Paul, J.; Wang, Y.-X. Emerging Role of MicroRNAs and Long Noncoding RNAs in Healthy and Diseased Lung. Adv. Exp. Med. Biol. 2017, 967, 343–359.
- Yu, B.; Wang, S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics 2018, 8, 3654–3675.
- Ju, C.; Liu, R.; Zhang, Y.-W.; Zhang, Y.; Zhou, R.; Sun, J.; Lv, X.-B.; Zhang, Z. Mesenchymal stem cell-associated lncRNA in osteogenic differentiation. Biomed. Pharmacother. 2019, 115, 108912.
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62.
- Shen, S.; Jiang, H.; Bei, Y.; Xiao, J.; Li, X. Long Non-Coding RNAs in Cardiac Remodeling. Cell. Physiol. Biochem. 2017, 41, 1830–1837.
- Sun, J.; Wang, C. Long non-coding RNAs in cardiac hypertrophy. Hearth Fail. Rev. 2020, 25, 1037–1045.
- Lorenzen, J.M.; Thum, T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 2016, 12, 360–373.
- Liu, Y.; Zhang, R.; Ying, K. Long non-coding RNAs: Novel links in respiratory diseases. Mol. Med. Rep. 2015, 11, 4025–4031.
- Zheng, Q.; Lin, Z.; Xu, J.; Lu, Y.; Meng, Q.; Wang, C.; Yang, Y.; Xin, X.; Li, X.; Pu, H.; et al. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting beta-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018, 9, 253.
- Gutschner, T.; Hämmerle, M.; Eißmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Groß, M.; et al. The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells. Cancer Res. 2013, 73, 1180–1189.
- Boucherat, O.; Vitry, G.; Trinh, I.; Paulin, R.; Provencher, S.; Bonnet, S. The cancer theory of pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 285–299.
- Jiang, M.-C.; Ni, J.-J.; Cui, W.-Y.; Wang, B.-Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019, 9, 1354–1366.
- Qin, Y.; Yan, G.; Qiao, Y.; Wang, D.; Luo, E.; Hou, J.; Tang, C. Emerging role of long non-coding RNAs in pulmonary hypertension and their molecular mechanisms. Exp. Ther. Med. 2020, 20, 1.
- Zahid, K.R.; Raza, U.; Chen, J.; Raj, U.J.; Gou, D. Pathobiology of pulmonary artery hypertension: Role of long non-coding RNAs. Cardiovasc. Res. 2020, 116, 1937–1947.
- Zehendner, C.M.; Valasarajan, C.; Werner, A.; Boeckel, J.N.; Bischoff, F.C.; John, D. Long Noncoding RNA TYKRIL Plays a Role in Pulmonary Hypertension via the p53-Mediated Regulation of PDGFRbeta. Am. J. Respir. Crit. Care Med. 2020, 116, 1937–1947.
- Omura, J.; Habbout, K.; Shimauchi, T.; Wu, W.-H.; Breuils-Bonnet, S.; Tremblay, E.; Martineau, S.; Nadeau, V.; Gagnon, K.; Mazoyer, F.; et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation 2020, 142, 1464–1484.
- Su, H.; Xu, X.; Yan, C.; Shi, Y.; Hu, Y.; Dong, L.; Ying, S.; Ying, K.; Zhang, R. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2018, 19, 1–18.
- Zhu, B.; Gong, Y.; Yan, G.; Wang, D.; Qiao, Y.; Wang, Q.; Liu, B.; Hou, J.; Li, R.; Tang, C. Down-regulation of lncRNA MEG3 promotes hypoxia-induced human pulmonary artery smooth muscle cell proliferation and migration via repressing PTEN by sponging miR-21. Biochem. Biophys. Res. Commun. 2018, 495, 2125–2132.
- Sun, Z.; Nie, X.; Sun, S.; Dong, S.; Yuan, C.; Li, Y. Long Non-Coding RNA MEG3 Downregulation Triggers Human Pulmonary Artery Smooth Muscle Cell Proliferation and Migration via the p53 Signaling Pathway. Cell Physiol. Biochem. 2017, 42, 2569–2581.
- Xing, Y.; Zheng, X.; Fu, Y.; Qi, J.; Li, M.; Ma, M.; Wang, S.; Li, S.; Zhu, D. Long Noncoding RNA-Maternally Expressed Gene 3 Contributes to Hypoxic Pulmonary Hypertension. Mol. Ther. 2019, 27, 2166–2181.
- Gong, J.; Chen, Z.; Chen, Y.; Lv, H.; Lu, H.; Yan, F.; Li, L.; Zhang, W.; Shi, J. Long non-coding RNA CASC2 suppresses pulmonary artery smooth muscle cell proliferation and phenotypic switch in hypoxia-induced pulmonary hypertension. Respir. Res. 2019, 20, 53.
- Jandl, K.; Thekkekara Puthenparampil, H.; Marsh, L.M.; Hoffmann, J.; Wilhelm, J.; Veith, C.; Kwapiszewska, G. Long non-coding RNAs influence the transcriptome in pulmonary arterial hypertension: The role of PAXIP1-AS. J. Pathol. 2019, 247, 357–370.
- Yang, L.; Liang, H.; Shen, L.; Guan, Z.; Meng, X. LncRNA Tug1 involves in the pulmonary vascular remodeling in mice with hypoxic pulmonary hypertension via the microRNA-374c-mediated Foxc1. Life Sci. 2019, 237, 116769.
- Wang, S.; Cao, W.; Gao, S.; Nie, X.; Zheng, X.; Xing, Y.; Chen, Y.; Bao, H.; Zhu, D. TUG1 Regulates Pulmonary Arterial Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Can. J. Cardiol. 2019, 35, 1534–1545.
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Günther, S.; et al. Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation 2017, 136, 65–79.
- Wang, S.; Zhang, C.; Zhang, X. Downregulation of long non-coding RNA ANRIL promotes proliferation and migration in hypoxic human pulmonary artery smooth muscle cells. Mol. Med. Rep. 2019, 21, 589–596.
- Zhang, H.; Liu, Y.; Yan, L.; Wang, S.; Zhang, M.; Ma, C.; Zhu, D. Long noncoding RNA Hoxaas3 contributes to hypoxia-induced pulmonary artery smooth muscle cell proliferation. Cardiovasc. Res. 2019, 115, 647–657.
- Wang, D.; Xu, H.; Wu, B.; Jiang, S.; Pan, H.; Wang, R.; Chen, J. Long non-coding RNA MALAT1 sponges miR-124-3p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int. J. Mol. Med. 2019, 44, 871–884.
- He, M.; Shen, J.; Zhang, C.; Chen, Y.; Wang, W.; Tao, K. Long-Chain Non-Coding RNA Metastasis-Related Lung Adenocarcinoma Transcript 1 (MALAT1) Promotes the Proliferation and Migration of Human Pulmonary Artery Smooth Muscle Cells (hPASMCs) by Regulating the MicroRNA-503 (miR-503)/Toll-Like Receptor 4 (TLR4) Signal Axis. Med. Sci. Monit. 2020, 26, 923123.
- Zhu, T.-T.; Sun, R.-L.; Yin, Y.-L.; Quan, J.-P.; Song, P.; Xu, J.; Zhang, M.-X.; Li, P. Long noncoding RNA UCA1 promotes the proliferation of hypoxic human pulmonary artery smooth muscle cells. Pflügers Arch. Eur. J. Physiol. 2018, 471, 347–355.
- Lei, S.; Peng, F.; Li, M.L.; Duan, W.B.; Peng, C.Q.; Wu, S.J. LncRNA-SMILR modulates RhoA/ROCK signaling by targeting miR-141 to regulate vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H377–H391.
- Liu, Y.; Sun, Z.; Zhu, J.; Xiao, B.; Dong, J.; Li, X. LncRNA-TCONS_00034812 in cell proliferation and apoptosis of pulmonary artery smooth muscle cells and its mechanism. J. Cell Physiol. 2018, 233, 4801–4814.
- Cheng, G.; He, L.; Zhang, Y. LincRNA-Cox2 promotes pulmonary arterial hypertension by regulating the let-7a-mediated STAT3 signaling pathway. Mol. Cell. Biochem. 2020, 475, 239–247.
- Liu, Y.; Zhang, H.; Li, Y.; Yan, L.; Du, W.; Wang, S.; Zheng, X.; Zhang, M.; Zhang, J.; Qi, J.; et al. Long Noncoding RNA Rps4l Mediates the Proliferation of Hypoxic Pulmonary Artery Smooth Muscle Cells. Hypertension 2020, 76, 1124–1133.
- Chen, J.; Guo, J.; Cui, X.; Dai, Y.; Tang, Z.; Qu, J.; Raj, J.U.; Hu, Q.; Gou, D. The Long Noncoding RNA LnRPT Is Regulated by PDGF-BB and Modulates the Proliferation of Pulmonary Artery Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2018, 58, 181–193.
- Liu, Y.; Hu, R.; Zhu, J.; Nie, X.; Jiang, Y.; Hu, P.; Liu, Y.; Sun, Z. The lncRNA PAHRF functions as a competing endogenous RNA to regulate MST1 expression by sponging miR-23a-3p in pulmonary arterial hypertension. Vascul. Pharmacol. 2021, 139, 106886.
- Hao, X.; Li, H.; Zhang, P.; Yu, X.; Jiang, J.; Chen, S. Down-regulation of lncRNA Gas5 promotes hypoxia-induced pulmonary arterial smooth muscle cell proliferation by regulating KCNK3 expression. Eur. J. Pharmacol. 2020, 889, 173618.
- Zhuo, Y.; Zeng, Q.; Zhang, P.; Li, G.; Xie, Q.; Cheng, Y. Functional polymorphism of lncRNA MALAT1 contributes to pulmonary arterial hypertension susceptibility in Chinese people. Clin. Chem. Lab. Med. 2017, 55, 38–46.
- Schlosser, K.; Hanson, J.; Villeneuve, P.J.; Dimitroulakos, J.; McIntyre, L.; Pilote, L.; Stewart, D.J. Assessment of Circulating LncRNAs Under Physiologic and Pathologic Conditions in Humans Reveals Potential Limitations as Biomarkers. Sci. Rep. 2016, 6, 36596.
- Smaldone, M.C.; Davies, B.J. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr. Opin. Mol. Ther. 2010, 12, 607–616.




