Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Erika Gress | + 2345 word(s) | 2345 | 2021-11-26 05:22:01 | | | |
| 2 | Beatrix Zheng | + 33 word(s) | 2378 | 2021-12-02 02:31:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gress, E. Symbiodiniaceae in Antipatharians (Black Corals). Encyclopedia. Available online: https://encyclopedia.pub/entry/16653 (accessed on 07 February 2026).
Gress E. Symbiodiniaceae in Antipatharians (Black Corals). Encyclopedia. Available at: https://encyclopedia.pub/entry/16653. Accessed February 07, 2026.
Gress, Erika. "Symbiodiniaceae in Antipatharians (Black Corals)" Encyclopedia, https://encyclopedia.pub/entry/16653 (accessed February 07, 2026).
Gress, E. (2021, December 01). Symbiodiniaceae in Antipatharians (Black Corals). In Encyclopedia. https://encyclopedia.pub/entry/16653
Gress, Erika. "Symbiodiniaceae in Antipatharians (Black Corals)." Encyclopedia. Web. 01 December, 2021.
Copy Citation
Antipatharians are understudied ecosystem engineers of shallow (<30 m depth), mesophotic (30–150 m) and deep-sea (>200 m) reefs. They provide habitat to numerous organisms, enhancing and supporting coral reef biodiversity globally. Nonetheless, little biological and ecological information exists on antipatharians, including the extent to which global change disturbances are threatening their health. The previous assumption that they were exempted from threats related to the phenomenon known as bleaching was challenged by the recent findings of high densities of dinoflagellates within three antipatharian colonies.
coral reefs
symbiotic algae
dinoflagellates
Madagascar
symbiosis
1. Introduction
In the coral–algae symbiotic association, Symbiodiniaceae harvest sunlight for photosynthesis and dissipate excess energy so as to prevent light-induced oxidative stress [1][2][3]. Under ambient conditions (i.e., not heat and light-stressed), Symbiodiniaceae absorbs light that can be (1) used to drive photochemistry, (2) re-emitted as fluorescence, (3) dissipated as heat or (4) decayed via the chlorophyll triplet state [1][2][3][4]. Experiments have shown that Symbiodiniaceae in scleractinians, under typical irradiances at shallow coral reefs (640 μmol photons m−2 s−1), dissipate 96% of the energy and use only 4% of the absorbed light energy for photosynthesis [1]. However, over prolonged periods of water temperature alterations, the invertebrate host needs to lower the number of its symbionts because the oxidative stress, which results from the production and accumulation of reactive oxygen species (ROS), can damage lipids, proteins and DNA [2][5]. When corals reduce the number of their symbionts, the main source of ROS production is removed, although the coral host itself may also produce ROS as a result of light and temperature [2]. Oxidative stress on corals results in a lack or low number of dinoflagellates and their photosynthetic pigments, an effect known as ‘coral bleaching’. Three potential mechanisms have been suggested for the regulation of symbiont numbers under such stressful conditions: (i) expulsion of excess symbionts; (ii) degradation of symbionts by host cells; and (iii) inhibition of symbiont cell growth and division controlled by the pH of the host cell [6][7].
The earliest suggestion of dinoflagellates being present in antipatharian tissues comes from the Report on the Antipatharia collected by H.M.S. Challenger by Brook [8]. A few years later, in a report on The Antipatharia of the Siboga Expedition, van Pesch [9] documented six species containing what he referred to as ‘symbiotic Algae’ ranging from 7–10 µm in diameter present in the gastrodermis. Significantly, he reported observing these cells in only six out of the thirty species he examined [9]). With no more empirical studies or reports for many decades and given antipatharians ability to thrive at abyssal depths and in low-light environments, they were assumed to lack Symbiodiniaceae, which is commonly referred to as ‘being azooxanthellate’ [10][11]. Moreover, a few later reports using molecular techniques did not find dinoflagellates in the antipatharian species examined. For instance, after intense morphological studies, dinoflagellates were reported absent in Antipathes grandis VERRILL, 1928, from Hawaii [12]. Likewise, dinoflagellate-specific primers and spectrophotometric methods that detect dinoflagellate chlorophyll absorbance patterns did not reveal any found microalgae in the species Stichopathes luetkeni (BROOK, 1889) (formerly called Cirrhipathes lutkeni) [13].
In contrast, in accordance with the early historical suggestions, two more recent studies have confirmed the presence of Symbiodiniaceae in various antipatharian species. A histological analysis of 14 antipatharian species collected from a depth between 10 and 396 m from Hawaii and Johnston Atoll revealed low densities (0–92 cells mm−3) of Symbiodiniaceae cells inside antipatharian gastrodermal tissues, suggesting that the dinoflagellates are endosymbiotic [11]. Additionally, dinoflagellates sequences retrieved from the antipatharians confirmed the presence of Symbiodiniaceae in the genera Cladocopium, Gerakladium and Durusdinium. However, it was concluded that the endosymbiotic dinoflagellates had no significant role in the ‘nutrition’ of the species examined and suggested more research to determine whether the association might be parasitic [11]. The conclusion was based on the low density of microalgae cells within the antipatharians and their presence in colonies at depths where light penetration does not enable photosynthesis. They did not find any pattern in the types of Symbiodiniaceae present in the different antipatharian species; therefore, they suggested that endosymbiont acquisition might occur opportunistically and not be host-specific. In another recent study conducted on a single species of the genus Cirrhipathes from Indonesia, two colonies were sampled at 38 m and one at 15 m and evidence of abundant (~107 cells cm–2) Symbiodiniaceae cells in the gastrodermis of the corals was found [14]. Among these, the authors identified two genera—Cladocopium and Gerakladium—the latter commonly found in association with clinoid sponges. They concluded that a mutualistic endosymbiosis existed based on the presence of the dinoflagellates inside the antipatharian gastrodermis and a symbiosome surrounding the microalgal cell, combined with evidence of its division inside the host [14]. These findings led us to reconsider our view of the vulnerability of antipatharians to global change and prompted us to further investigate the occurrence of dinoflagellate symbionts in antipatharian species.
Most lineages in the subclass Hexacorallia are believed to have evolved photosymbioses independently [15], with the order Antipatharia being one of the exceptions until evidence of antipatharian species hosting dinoflagellates inside the coral gastrodermis was found [11][14]. However, these two studies presented two contrasting conclusions. In one case, it was concluded that the presence of the Symbiodiniaceae was opportunistic, and in the other, it was concluded that a mutualistic endosymbiosis existed. Those conclusions were based on the presence and abundance of the Symbiodiniaceae cells and their location in the host tissue, although from a very limited number of specimens. The present study was therefore undertaken with the objective of gaining further insight into the possibility of antipatharians hosting high abundances of dinoflagellates, expanding the geographic range of the species studied and the number of colonies examined. An integrated methodological approach was used, combining both microscopy and molecular techniques to investigate the presence, abundance, location and identity of Symbiodiniaceae in two antipatharian species—Cupressopathes abies (LINNAEUS, 1758) and Stichopathes maldivensis COOPER, 1903. The samples represent two different morphologies and were collected from both shallow and mesophotic reefs in SW Madagascar.
2. Overall Analysis Results
Despite photosymbiosis being evolutionarily conserved within most lineages in the subclass Hexacorallia [15], an endosymbiotic association between antipatharians and Symbiodiniaceae has not been clearly established. High densities of dinoflagellates (~107 cells cm–2) were found in three whip-like colonies (Cirrhipathes sp.) [14]. Therefore, it was considered that a study involving a higher number of samples was required in order to assess the microalgal presence and estimate their densities in a greater number of samples from more species and to identify possible patterns regarding species, colony morphology and depth. The present study showed either none or only very low densities (0–4 cells mm−3) of Symbiodiniaceae-like cells in both the whip-like S. maldivensis and the bushy C. abies, both on shallow and on mesophotic reefs. These new findings align with both previous historical observations and more subsequent studies that found few, if any, endosymbiotic algae in antipatharians [8][9][11][12][13]. These studies—ours included—evidence that high abundances of dinoflagellates within antipatharians is not a common finding. Considering the relevance of antipatharians for habitat provision on reefs and the increasing extent of mass bleaching events affecting scleractinian corals, the possibility of most antipatharians being exempt from this threat warrants further investigation.
While, in our study, the dinoflagellates’ identity could not be determined by molecular analysis, the Symbiodiniaceae-like cells observed in the histological sections of the whip-like S. maldivensis closely resembled those described in the other two studies [11][14] (Figure 1). For future histological examination of antipatharians, where the tissue cannot be separated from the skeleton (such as C. abies), we suggest experimenting with softening the skeletons with a lytic polysaccharide monooxygenases (LPMOs) treatment [16]. We applied the same sodium hydroxide isolation protocol—a protocol found to be effective for scleractinians and other cnidarians [17]—to a scleractinian coral fragment, and it was clear that the cells obtained were Symbiodiniaceae (Figure 2). Nevertheless, it is probably safer to refer to the cells in our antipatharian samples only as being Symbiodiniaceae-like.
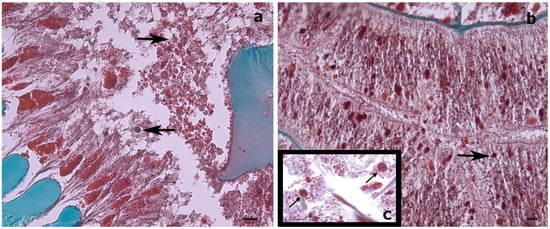
Figure 1. Histological cross-sections of a Stichopathes maldivensis polyp. Symbiodiniaceae-like cells (arrows) are observed in the gastrodermis of the gastrovascular cavity (a) and in the gastrodermis of a lateral tentacle (b). (c) Inset for comparison is an image from Wagner et al. (2011, Figure 3c) in which Symbiodiniaceae cells were identified through histological examination and molecular analysis. Scale bars = 20 μm.
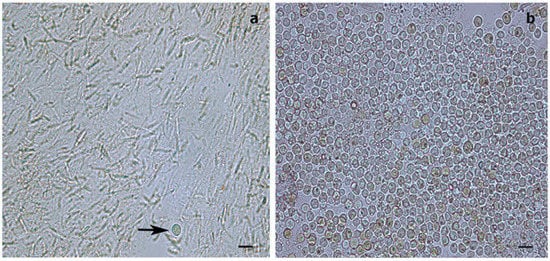
Figure 2. Light microscopy images of Symbiodiniaceae-like extracts obtained through the sodium hydroxide isolation method. (a) Extract from the antipatharian Cupressopathes abies showing only one Symbiodiniaceae-like cell (arrow) but abundant cnidocytes. (b) Extract from the scleractinian Seriatopora hystrix showing numerous Symbiodiniaceae-like cells, corroborating the efficiency of the isolation method. Scale bars = 10 μm.
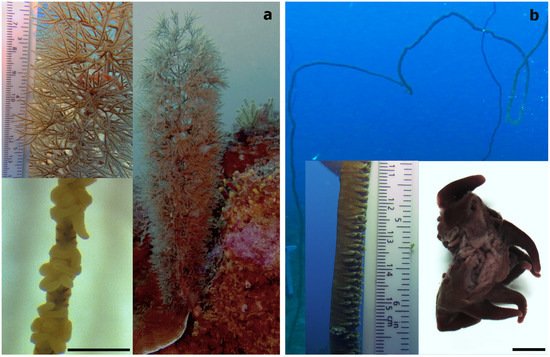
Figure 3. (a) The bottle-brush-like Cupressopathes abies and (b) the whip-like Stichopathes maldivensis, with an image showing a section of two individual polyps in the bottom right corner. Scale bars of polyp images = 1 mm.
In the present study, Symbiodiniaceae-like cells were observed in the SEM and histological examination located inside the coral gastrodermis of S. maldivensis (Figure 4 and Figure 5). This suggests an endosymbiotic association, which was also suggested from histological sections analysis and from ultrastructural analysis [11][14]. The fact that no Symbiodiniaceae ITS2 sequences were amplified from the DNA extractions in this study seems most likely due to the extremely low density of dinoflagellates. In the two studies where identification was possible, different genera (Cladocopium, Gerakladium and Durusdinium) were reported to be associated with different antipatharian species [11][14]. Such plasticity, even at the intra-colony level, has also been evidenced in more recent studies on scleractinian corals and is believed to be environmentally driven [18]. Moreover, there is evidence to suggest that the same Symbiodiniaceae species may be mutualistic in one host context but opportunistic in another [19]. Therefore, the identity of the dinoflagellates alone is insufficient to determine the type of symbiosis. Further studies will be necessary to determine the nature of the association between Symbiodiniaceae and antipatharians, particularly in cases where microalgae abundance is high, as in [14].
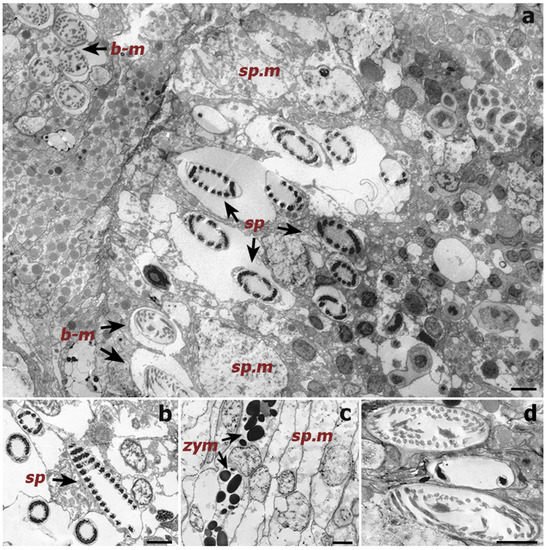
Figure 4. Transmission electron microscope (TEM) images of a cross-section of a Cupressopathes abies polyp tentacle. (a) Ultrastructural view of numerous cnidocytes, including: spirocysts (sp) and b-mastigophores (b-m), as well as spumous mucous cells (sp.m). (b) Longitudinal section of mature spirocysts (sp). (c) Close view of zymogen granules (zym) and spumous mucous cells (sp.m) inside the gastrodermis. (d) Cross-section of mature b-mastigophores. Scale bars = 2 μm.
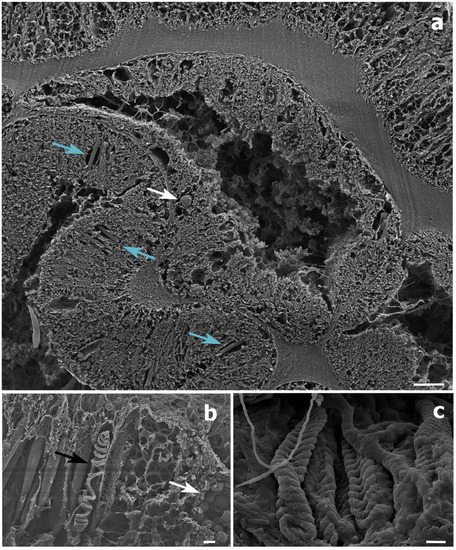
Figure 5. Scanning electron microscope (SEM) images of a Stichopathes maldivensis polyp. (a) A round potentially dinoflagellate-cell of about 8 μm inside the gastrodermis of the polyp gastrovascular cavity (white arrow). Numerous b-mastigophores can be observed in the ectoderm (blue arrows). (b) Smaller round cells (3–4 μm) that are likely to be mucous cells (white arrow) in the polyp ectoderm. The image also shows several b-mastigophores aligned among which one has a broken capsule which exposes the cnidocyte tubule (black arrow). (c) Closer view of spirocysts in a polyp tentacle ectoderm. Scale bars: a = 10 μm, b = 2 μm, c =1 μm.
3. Dinoflagellate Density Difference between Species
Density estimates showed that colonies of the whip-like S. maldivensis had significantly more Symbiodiniaceae-like cells within their tissues compared to C. abies regardless of depth (0.084 mean cells mm−3 density difference, Table 1). However, due to the very low densities of dinoflagellates observed in both species, it is difficult to determine the biological significance of this difference. To date, a single study examined multiple species (14) and different colony morphologies (both whip-like and branching) [11]. The branching species, Antipathes griggi, recorded the highest density (0–92 cells mm−3), although microalgal cells were observed in only one of the eight colonies of this species examined. Variation in density was also observed in our study, which is the first to have examined several colonies of the same species from two contrasting depths. Of the eleven colonies of each species, only four colonies of C. abies and seven of S. maldivensis were observed to contain microalgae. On the other hand, no differences were found between the three different regions (top, middle and base) within any single colony. This suggests that the intra-specific variability recorded in [11], as well as ourselves, is unlikely to be caused by variations in the microalgal cell density between different parts of the colonies. From this limited information, it seems plausible that other factors, rather than species or morphology, might account for the greater numbers of microalgal cells found in some antipatharian colonies.
Table 1. Results of the quasi-Poisson GLM test for differences between species and depths. The intercept represents C. abies dinoflagellate cell density. Significant p-value (p < 0.05) are shown in bold.
| Factor | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | −2.27 | 0.74 | −3.08 | 0.006 |
| S. maldivensis | 1.16 | 0.49 | 2.36 | 0.029 |
| Depth | −0.04 | 0.02 | −1.58 | 0.131 |
4. Dinoflagellate Density Difference between Depths
Contrary to expectation, no statistically significant difference in dinoflagellate density was found between shallow and mesophotic depths, although the mean dinoflagellates density at 20 m depth was greater than at 40 m depth in both species (the mean differences between depths being 0.200 and 0.016 cells mm−3 for S. maldivensis and C. abies, respectively). Differences in microalgae density can be inferred from previous studies. For instance, among the 14 samples from different colonies of A. griggi from Hawaii for which the cell density was reported, the highest densities were from the two colonies sampled on shallow reefs [11]. The samples of Cirrhipathes collected in Bunaken, Indonesia, were from two different depths (15 and 38 m) [14]. While it was not reported whether there were any differences in Symbiodiniaceae density related to depth, the Kd 490 (proxy of turbidity) values at 15 m and 38 m depth in Bunaken have been estimated to be very similar [19]. In Toliara, Madagascar, where the Kd 490 values indicate high turbidity (Figure 6b) due to sedimentation derived from river runoff, light penetration at a 40 m depth is likely to be considerably reduced (as was noticeable during field work). However, it is not inevitable that higher densities of Symbiodiniaceae should be found in association with antipatharians in shallower water since it is believed that the uptake of dinoflagellates in the coral–algae symbiotic association can be controlled by the coral host [6][7]. More studies on antipatharian colonies exposed to higher radiations are therefore necessary to assess the significance of high densities of dinoflagellates in antipatharians (assuming they occur), and to understand the mechanism behind the association.
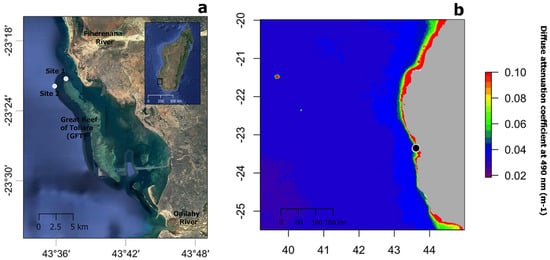
Figure 6. (a) Map showing the location of study sites 1 and 2 (white circles) near the Great Reef of Toliara (GRT) in SW Madagascar. (b) Annual median of the diffuse attenuation coefficient for downwelling irradiance at 490 nm in m−1 (Kd 490) for the year 2018 over the wider region (black circle indicated the location of the GRT). Satellite data derived from MODIS-Aqua accessed through OceanColor (https://oceancolor.gsfc.nasa.gov, accessed on 2 April 2019). Increasing coefficient values indicate higher water turbidity.
References
- Brodersen, K.E.; Lichtenberg, M.; Ralph, P.; Kühl, M.; Wangpraseurt, D. Radiative energy budget reveals high photosynthetic efficiency in symbiont-bearing corals. J. R. Soc. Interface 2014, 11, 20130997.
- Roth, M.S. The engine of the reef: Photobiology of the coral-algal symbiosis. Front. Microbiol. 2014, 5, 422.
- Roth, M.; Padilla-Gamiño, J.; Pochon, X.; Bidigare, R.; Gates, R.; Smith, C.; Spalding, H. Fluorescent proteins in dominant mesophotic reef-building corals. Mar. Ecol. Prog. Ser. 2015, 521, 63–79.
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066.
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278.
- Davy, S.K.; Allemand, D.; Weis, V. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol Rev. 2012, 76, 229–261.
- Barott, K.L.; Venn, A.A.; Perez, S.O.; Tambutté, S.; Tresguerres, M. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 607–612.
- Brook, G. Report on the Antipatharia collected by HMS Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of HMS Challenger During the Years 1873–76. Zoology 1889, 32, 1–222.
- van Pesch, A.J. The Antipatharia of the Siboga Expedition. Siboga Exped. Monogr. 1914, 17, 1–258.
- Grigg, R.W. Ecological studies of Black Coral in Hawaii. Pac. Sci. 1965, 19, 244–260.
- Wagner, D.; Pochon, X.; Irwin, L.; Toonen, R.; Gates, R.D. Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proc. R. Soc. B Biol. Sci. 2010, 278, 1323–1328.
- Grigg, R.W. A Contribution to the Biology and Ecology of the Black Coral, Antipathes grandis in Hawai‘i. MS Thesis in Zoology, 1964, p. 74. Hawai‘i., Honolulu. Available online: http://cn.deziderkostrec.xyz/read/?id=Qv7PHAAACAAJ&format=pdf&server=1 (accessed on 2 April 2019).
- Santiago-Vázquez, L.Z.; Brück, T.B.; Brück, W.M.; Duque-Alarcón, A.P.; McCarthy, P.J.; Kerr, R.G.; Br, T.B. The diversity of the bacterial communities associated with the azooxanthellate hexacoral Cirrhipathes lutkeni. ISME J. 2007, 1, 654–659.
- Bo, M.; Baker, A.; Gaino, E.; Wirshing, H.; Scoccia, F.; Bavestrello, G. First description of algal mutualistic endosymbiosis in a black coral (Anthozoa: Antipatharia). Mar. Ecol. Prog. Ser. 2011, 435, 1–11.
- McFadden, C.S.; Quattrini, A.M.; Brugler, M.R.; Cowman, P.F.; Dueñas, L.F.; Kitahara, M.V.; A. Paz-García, D.; Reimer, J.D.; Pichon, M. Recherches sur les peuplements à dominance d’anthozoaires dans les récifs coralliens de Tuléar (Madagascar). Atoll Res. Bull. 1978, 222, 1–490.
- Pettay, D.T.; Wham, D.C.; Smith, R.T.; Iglesias-Prieto, R.; LaJeunesse, T.C. Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc. Natl. Acad. Sci. USA 2015, 112, 7513–7518.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 2 April 2019).
- Meistertzheim, A.-L.; Pochon, X.; Wood, S.A.; Ghiglione, J.-F.; Hédouin, L. Development of a quantitative PCR–high-resolution melting assay for absolute measurement of coral-Symbiodiniaceae associations and its application to investigating variability at three spatial scales. Mar. Biol. 2019, 166, 13.
- Holden, H. Characterisation of Optical Water Quality in Bunaken National Marine Park, Indonesia. Singap. J. Trop. Geogr. 2002, 23, 23–36.
More
Information
Subjects:
Agriculture, Dairy & Animal Science; Area Studies
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
955
Revisions:
2 times
(View History)
Update Date:
02 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




