Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chunpeng Wan | + 1946 word(s) | 1946 | 2021-11-26 10:20:29 | | | |
| 2 | Vivi Li | Meta information modification | 1946 | 2021-11-29 02:14:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wan, C. Aloe vera Gel. Encyclopedia. Available online: https://encyclopedia.pub/entry/16447 (accessed on 07 February 2026).
Wan C. Aloe vera Gel. Encyclopedia. Available at: https://encyclopedia.pub/entry/16447. Accessed February 07, 2026.
Wan, Chunpeng. "Aloe vera Gel" Encyclopedia, https://encyclopedia.pub/entry/16447 (accessed February 07, 2026).
Wan, C. (2021, November 26). Aloe vera Gel. In Encyclopedia. https://encyclopedia.pub/entry/16447
Wan, Chunpeng. "Aloe vera Gel." Encyclopedia. Web. 26 November, 2021.
Copy Citation
Edible coating gels developed from the Aloe vera plant have been used as a traditional medicine for about 3000 years. Aloe vera contains approximately 110 potentially active constituents from six different classes: chromone and its glycoside derivatives; anthraquinone and its glycoside derivatives; flavonoids; phenylpropanoids and coumarins; phenylpyrone and phenol derivatives; and phytosterols and others. Apart from medicinal uses, Aloe gels have an important role in food preservation as edible coatings. They provide an edible barrier for atmospheric gases and moisture and help to reduce the respiration and transpiration of fresh produce, which helps to preserve its postharvest quality.
Aloe vera
chemical constituents
antimicrobial activity
postharvest storage
biodegradable
edible coating
1. Introduction
Food quality mainly refers to three attributes: external (size, color, appearance, etc.), internal (taste, color, juicy, texture, seedless, etc.), and hidden (food safety and nutritional contents). External and internal quality attributes were of greatest importance to consumers for many years. However, since the occurrence of food-derived health problems has begun to increase, consumers have started to pay more attention to the hidden quality attributes of fresh produce and are asking for food to be free of chemical residues [1]. Fungicides and other agrochemicals are of great importance in controlling postharvest diseases and have crucial role for the preservation of the postharvest quality, but misuse and/or excessive use of them might cause negative impacts on human health [2][3]. There is an increasing effort in postharvest studies to develop natural preservatives and antimicrobials to extend the storage duration of foods without chemical preservatives [4][5]. So far, many storage techniques and natural preservatives have been developed to extend the postharvest life of foods. The currently utilized natural preservatives are chitosan [6][7], essential oils [8][9], propolis extract [10], plant extracts [11][12], edible coatings [13][14], and organic salts [15]. Among these, edible coatings have been receiving more attention in recent years due to their potential for developing edible packaging materials [16]. Weight loss, changes in textural quality, changes in chemical structure, and microbial pathogens (mostly fungus) are the most important postharvest problems for foods [17][18].
Aloe belongs to the family of Xanthorrhoeaceae, which consists of about 420 species, and has been used as a traditional medicine for about 3000 years [19]. The perennial plant known as Aloe vera is Aloe barbadensis Miller, which is a well-known pharmaceutical herb that has long been used in traditional Chinese medicine for the treatment of various diseases. It is widely distributed in the semitropical regions and cultivated in many provinces of China.
2. Chemical Constituents of Aloe vera
The two-main class active constituent of the Aloe vera plant extract are chromone and anthraquinone and its glycoside derivatives, alongside others such as phenylpyrone derivatives, flavonoids, phenylpropanoids, coumarins, phytosterols, naphthalene analogs, lipids, and vitamins.
2.1. Chromone and its Glycoside Derivatives
Approximately 29 chromone derivatives were isolated and identified from Aloe vera (Table 1, Figure 1). Aloesin (1, formerly called aloeresin B), aloeresin A (23), isoaloeresin D (13) and aloeresin E (9) are the most significant active constituents of Aloe vera. Three aloediols (7, 8, and 9) were isolated and identified from Aloe vera, but the absolute configuration has not yet been determined.
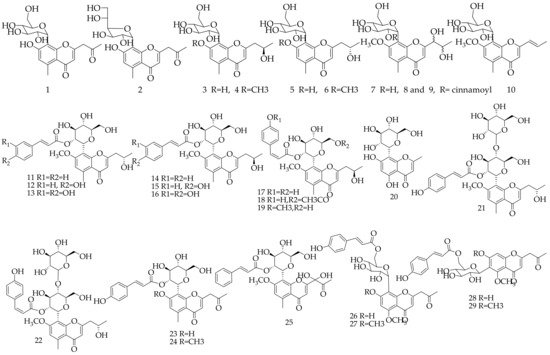
Figure 1. Chemical structure of chromone and its glycoside derivatives from Aloe vera.
Table 1. Chromone and its glycoside derivatives isolated and identified from Aloe vera.
| No | Constituents | Molecular Formula | Exact Mass | References |
|---|---|---|---|---|
| 1 | aloesin | C19H22O9 | 394.1264 | [20][21] |
| 2 | neoaloesin A | C19H22O9 | 394.1264 | [22] |
| 3 | 8-C-glucosyl-(R)-aloesol | C19H24O9 | 396.142 | [20] |
| 4 | 8-C-glucosyl-7-methoxy-(R)-aloesol | C20H26O9 | 410.1577 | [20] |
| 5 | 8-C-glucosyl-(S)-aloesol | C19H24O9 | 396.142 | [23] |
| 6 | 8-C-glucosyl-7-methoxy-(S)-aloesol | C20H26O9 | 410.1577 | [23][24] |
| 7 | 8-C-glucosyl-7-O-methylaloediol | C20H26O10 | 426.1526 | [20][23] |
| 8 | 8-glucosyl-(2’-O-cinnamoyl)-7-O-methylaloediol A | C29H32O12 | 572.1894 | [25] |
| 9 | 8-glucosyl-(2’-O-cinnamoyl)-7-O-methylaloediol B | C29H32O12 | 572.1894 | [25] |
| 10 | C-2′-decoumaroyl-aloeresin G | C20H24O8 | 392.1471 | [20] |
| 11 | aloeresin E | C29H32O10 | 540.1995 | [24] |
| 12 | isoaloeresin D | C29H32O11 | 556.1945 | [24][26][27] |
| 13 | iso-rabaichromone | C29H32O12 | 572.1894 | [23] |
| 14 | 8-[C-β-D-[2-O-(E)-cinnamoyl] glucopyranosyl]-2-[(R)-2-hydroxypropyl]-7-methoxy-5-methylchromone | C29H32O10 | 540.1995 | [28] |
| 15 | aloeresin D | C29H32O11 | 556.1945 | [20][28] |
| 16 | rabaichromone | C29H32O12 | 572.1894 | [20] |
| 17 | allo-aloeresin D | C29H32O11 | 556.1945 | [20] |
| 18 | aloeresin K | C31H34O12 | 598.205 | [27] |
| 19 | aloeresin J | C30H34O11 | 570.2101 | [27] |
| 20 | 8-C-glucosyl-noreugenin | C16H18O9 | 354.0951 | [25] |
| 21 | 4’-O-glucosyl-isoaloeresin DI | C35H42O16 | 718.2473 | [25] |
| 22 | 4’-O-glucosyl-isoaloeresin DII | C35H42O16 | 718.2473 | [25] |
| 23 | aloeresin A | C28H28O11 | 540.1632 | [21] |
| 24 | 7-O-methyl-aloeresin A | C29H30O11 | 554.1788 | [21][29] |
| 25 | 9-dihydroxyl-2’-O-(Z)-cinnamoyl-7-methoxy-aloesin | C29H30O12 | 570.1737 | [29] |
| 26 | 6′-O-coumaroyl-aloesin | C28H28O12 | 556.1581 | [30] |
| 27 | 7-methoxy-6′-O-coumaroyl-aloesin | C29H30O12 | 570.1737 | [31] |
| 28 | aloeveraside B | C28H28O12 | 556.1581 | [30][32] |
| 29 | aloeveraside A | C29H30O12 | 570.1737 | [30][32] |
2.2. Anthraquinone and its Glycoside Derivatives
Approximately 32 anthraquinones and their glycoside derivatives were isolated and identified from Aloe vera (Table 2, Figure 2). The isomers of aloin A (30) and aloin B (31), two anthraquinone glucosides, are the most abundant active constituents of Aloe vera. However, chrysophanol (52), emodin (53), physcione (54), aloe-emodin (55) are four major anthraquinone aglycones. Six anthraquinone dimmers (45–50) were also identified from Aloe vera.
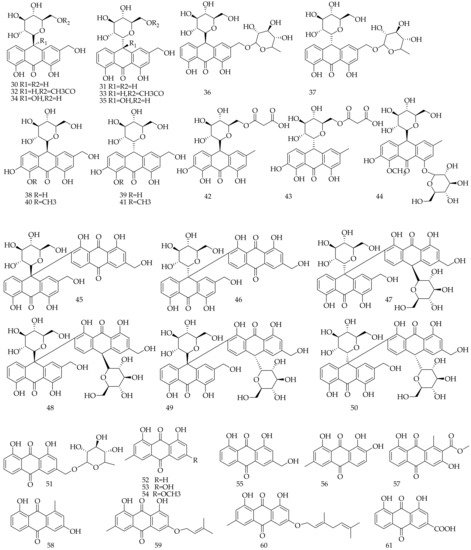
Figure 2. Chemical structure of anthraquinone and its glycoside derivatives from Aloe vera.
Table 2. Anthraquinone and its glycoside derivatives isolated and identified from Aloe vera.
| No | Constituents | Molecular Formula | Exact Mass | References |
|---|---|---|---|---|
| 30 | aloin A | C21H22O9 | 418.1264 | [27] |
| 31 | aloin B | C21H22O9 | 418.1264 | [27] |
| 32 | 6′-O-acetyl-aloin A | C23H24O10 | 460.1369 | [27] |
| 33 | 6′-O-acetyl-aloin B | C23H24O10 | 460.1369 | [27] |
| 34 | 10-hydroxyaloins A | C21H22O10 | 434.1213 | [26][30] |
| 35 | 10-hydroxyaloins B | C21H22O10 | 434.1213 | [26][30] |
| 36 | aloinoside A | C27H32O13 | 564.1843 | [27] |
| 37 | aloinoside B | C27H32O13 | 564.1843 | [27] |
| 38 | 7-hydroxyaloin A | C21H22O10 | 434.1213 | [21] |
| 39 | 7-hydroxyaloin B | C21H22O10 | 434.1213 | [21] |
| 40 | 7-hydroxy-8-O-methylaloin A | C22H24O10 | 448.1369 | [21][26] |
| 41 | 7-hydroxy-8-O-methylaloin B | C22H24O10 | 448.1369 | [21][26] |
| 42 | 6′-malonylnataloin A | C24H24O12 | 504.1268 | [21] |
| 43 | 6′-malonylnataloin B | C24H24O12 | 504.1268 | [21] |
| 44 | homonataloside B | C28H34O14 | 594.1949 | [21] |
| 45 | elgonica dimer A | C36H30O14 | 686.1636 | [27][33][34] |
| 46 | elgonica dimer B | C36H30O14 | 686.1636 | [27][33][34] |
| 47 | aloindimer A | C42H42O18 | 834.2371 | [27] |
| 48 | aloindimer B | C42H42O18 | 834.2371 | [27] |
| 49 | aloindimer C | C42H42O18 | 834.2371 | [27] |
| 50 | aloindimer D | C42H42O18 | 834.2371 | [27] |
| 51 | aloe-emodin-11-O-rhamnoside | C21H20O9 | 416.1107 | [30] |
| 52 | chrysophanol | C15H10O4 | 254.0579 | [35] |
| 53 | emodin | C15H10O5 | 270.0528 | [30][35] |
| 54 | physcione | C16H12O5 | 284.0685 | [35] |
| 55 | aloe-emodin | C15H10O5 | 270.0528 | [30][35] |
| 56 | nataloeemodin | C15H10O5 | 270.0528 | [21] |
| 57 | aloesaponarin I | C17H12O6 | 312.0634 | [36] |
| 58 | aloesaponarin II | C15H10O4 | 254.0579 | [36] |
| 59 | madagascine | C20H18O5 | 338.1154 | [37] |
| 60 | 3-Geranyloxyemodin | C24H24O5 | 392.1624 | [37] |
| 61 | rhein | C15H8O6 | 284.0321 | [35] |
2.3. Flavonoids
Approximately 13 flavonoids and their glycoside derivatives were isolated and identified from Aloe vera (Table 3, Figure 3), including three types; namely flavone (62–67), flavonol (68–72), and flavan-3-ol (73,74).
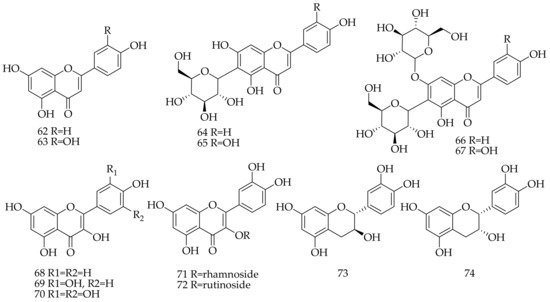
Figure 3. Chemical structure of flavonoids from Aloe vera.
Table 3. Flavonoids isolated and identified from Aloe vera.
| No | Constituents | Molecular formula | Exact Mass | References |
|---|---|---|---|---|
| 62 | apigenin | C15H10O5 | 270.0528 | [38] |
| 63 | luteolin | C15H10O6 | 286.0477 | [39] |
| 64 | isovitexin | C21H20O10 | 432.1056 | [39] |
| 65 | isoorientin | C21H20O11 | 448.1006 | [39] |
| 66 | saponarin | C27H30O15 | 594.1585 | [39] |
| 67 | lutonarin | C27H30O16 | 610.1534 | [39] |
| 68 | kaempferol | C15H10O6 | 286.0477 | [38] |
| 69 | quercetin | C15H10O7 | 302.0427 | [38] |
| 70 | myricetin | C15H10O8 | 318.0376 | [38] |
| 71 | quercitrin | C21H20O11 | 448.1006 | [38] |
| 72 | rutin | C27H30O16 | 610.1534 | [38] |
| 73 | catechin | C15H14O6 | 290.0790 | [38] |
| 74 | epicatechin | C15H14O6 | 290.0790 | [38] |
2.4. Phenylpropanoids and Coumarins
Approximately 12 phenylpropanoid acids and their ester derivatives (75–86), and four coumarins (87–90), were isolated and identified from Aloe vera (Table 4, Figure 4).
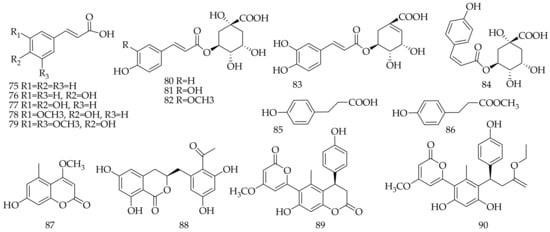
Figure 4. Chemical structure of phenylpropanoids and coumarins from Aloe vera.
Table 4. Phenylpropanoids and coumarins isolated and identified from Aloe vera.
| No | Constituents | Molecular Formula | Exact Mass | References |
|---|---|---|---|---|
| 75 | cinnamic acid | C9H8O2 | 148.0524 | [40] |
| 76 | p-coumaric | C9H8O3 | 164.0473 | [38] |
| 77 | caffeic acid | C9H8O4 | 180.0423 | [38] |
| 78 | ferulic acid | C10H10O4 | 194.0579 | [38] |
| 79 | sinapic acid | C11H12O5 | 224.0685 | [38] |
| 80 | 5-p-coumaroylquinic | C16H18O8 | 338.1002 | [39] |
| 81 | chlorogenic | C16H18O9 | 354.0951 | [38] |
| 82 | 5-feruloylquinic | C17H20O9 | 368.1107 | [39] |
| 83 | caffeoylshikimic | C16H16O8 | 336.0845 | [39] |
| 84 | 5-p-cis-coumaroylquinic | C16H18O8 | 338.1002 | [39] |
| 85 | 3-(4-hydroxyphenyl) propanoic acid | C9H10O3 | 166.063 | [30] |
| 86 | methyl 3-(4-hydroxyphenyl) propionate | C10H12O3 | 180.0786 | [30] |
| 87 | 7-demethylsiderin | C11H10O4 | 206.0579 | [30] |
| 88 | feralolide | C18H16O7 | 344.0896 | [31][34] |
| 89 | dihydrocoumarin | C22H18O7 | 394.1053 | [41] |
| 90 | dihydrocoumarin ethyl ester | C25H26O7 | 438.1679 | [41] |
2.5. Phenylpyrone and Phenol Derivatives
Approximately three phenylpyrone derivatives (91–93), one triglucosylated naphthalene derivative named aloveroside A (94), and one 1-methyltetralin derivative feroxidin (95) were isolated and identified from Aloe vera (Table 5, Figure 5). Nine phenol derivatives (96–104) and vitamin C (105) were also isolated from Aloe vera.
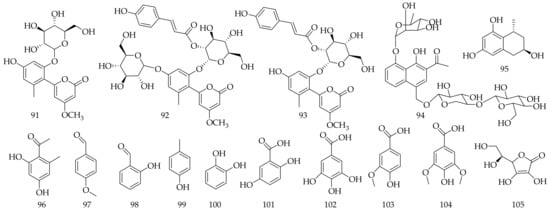
Figure 5. Chemical structure of phenylpyrone and phenol derivatives from Aloe vera.
Table 5. Phenylpyrone and phenol derivatives isolated and identified from Aloe vera.
| No | Constituents | Molecular Formula | Exact Mass | References |
|---|---|---|---|---|
| 91 | aloenin A | C19H22O10 | 410.1213 | [42] |
| 92 | aloenin B | C34H38O17 | 718.2109 | [33][42] |
| 93 | p-coumaroyl aloenin | C28H28O12 | 556.1581 | [33] |
| 94 | aloveroside A | C30H40O17 | 672.2265 | [33] |
| 95 | feroxidin | C11H14O3 | 194.0943 | [30] |
| 96 | 1-(2,4-dihydroxy-6-methylphenyl) ethanone | C9H10O3 | 166.0630 | [30] |
| 97 | p-anisaldehyde | C8H8O2 | 136.0524 | [30] |
| 98 | salicylaldehyde | C7H6O2 | 122.0368 | [30] |
| 99 | p-cresol | C7H8O | 108.0575 | [30] |
| 100 | pyrocatechol | C6H6O2 | 110.0368 | [40] |
| 101 | gentisic acid | C7H6O4 | 154.0266 | [38] |
| 102 | gallic acid | C7H6O5 | 170.0215 | [38] |
| 103 | vanillic acid | C8H8O4 | 168.0423 | [38] |
| 104 | syringic acid | C9H10O5 | 198.0528 | [38] |
| 105 | ascorbic acid | C6H8O6 | 176.0321 | [38] |
2.6. Phytosterols and Others
Five phytosterols (Table 6, Figure 6) were isolated from Aloe vera gel, including cycloartanol (106), 24-methylene-cycloartanol (107), lophenol (108), 24-methyl-lophenol (109), and 24-ethyl-lophenol (110) [43]. Some polar and nonpolar lipids, as well as prostanoids, were also isolated from Aloe vera leaves [44]. Chemical investigation of the major constituents in Aloe vera leaves revealed moisture, ash, fiber, protein, lipids, minerals, organic acids, free sugars, and polysaccharides. Glucose, fructose, and sucrose were the main free sugars. Oxalic, L-Malic, isocitric, lactic, acetic, isocitric, lactone, citric, and fumaric acid were the main organic acids.
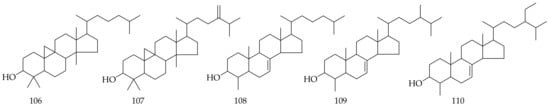
Figure 6. Chemical structure of phytosterols from Aloe vera.
Table 6. Phytosterols isolated and identified from Aloe vera.
| No | Constituents | Molecular Formula | Exact Mass | References |
|---|---|---|---|---|
| 106 | cycloartanol | C30H52O | 428.4018 | [43] |
| 107 | 24-methylene-cycloartanol | C31H52O | 440.4018 | [43] |
| 108 | lophenol | C28H48O | 400.3705 | [43] |
| 109 | 24-methyl-lophenol | C29H50O | 414.3862 | [43] |
| 110 | 24-ethyl-lophenol | C30H52O | 428.4018 | [43] |
3. Antimicrobial Activity of Aloe vera
Aloe vera plant extracts have antimicrobial characteristics that kill microorganisms (including bacteria (antibacterial activity), fungi (antifungal activity), and viruses (antiviral activity)) or stop their growth. Fruit decay is an important parameter influencing the postharvest quality of fresh produce. Previous studies have shown that the use of Aloe vera gel as an edible coating has positive effects on the prevention of fruit decay and microbial spoilage. The inhibitory effects of Aloe vera gel on the growth of mycelium (Penicillium digitatum and Aspergillus niger) was reported by Nabigol and Asghari [45], who performed a range of laboratory tests. They suggested that the inhibition of the mycelium growth rate increased with gel concentration. The 500 mL/L dose of Aloe vera gel was found to cause 100% inhibition of P. digitatum and 64% of A. niger. According to the findings of Kator et al. [46], 20%, 60%, and 100% concentrations of Aloe vera gel are effective in preventing the occurrence of decay in tomato fruits for seven days of storage. However, these authors suggested that a 100% concentration has significantly higher effects, and the positive impact may continue for 16 days of storage. Benitez et al. [47] reported that Aloe vera gel provides higher efficacy for the prevention of mesophilic bacteria and yeasts and molds than alginate and chitosan for kiwifruit slices. In another study, the shelf life of guava was reported to be increased by about one more week with the application of an Aloe vera gel coating, due to the fact that the edible coating prevents microbial growth [48]. Sitara et al. [49] conducted a comprehensive study regarding the antifungal activity of Aloe vera gel at three different doses against five plant pathogenic fungi: A. niger, Aspergillus flavus, Alternaria alternata, Drechslera hawaiensis, and P. digitatum. The highest test dose (0.35%) of Aloe vera gel was reported to completely inhibit the growth of Drechslera hawaiensis and Alternaria alternata. In another study, the minimum fungicidal concentrations of Aloe vera against Botrytis gladiolorum, Fusarium oxysporum f.sp. gladioli, Heterosporium pruneti, and Penicillium gladioli were reported to vary between 80 and 100 μL/mL, depending on the fungal species [50].
Previous studies have also shown that the combination of Aloe vera gel with some homogenizers, such as glycerol starch (0.15 g), improves the efficacy in controlling fungal decay and weight loss in cherry tomatoes [51]. The specific mechanism of action is still unknown, but it is known that saponins, acemannan and anthraquinone derivatives, which are found in Aloe vera, have antibacterial activity [52]. Navarro et al. [53] performed a study with Aloe vera gel alone or in combination with thymol on nectarines and reported that the Aloe vera gel alone is more efficient in prevention of the decay caused by Rhizopus stolonifer, B. cinerea, and P. digitatum. Aloe vera gel coatings were previously tested against decay and found to significantly lower counts for molds, yeast, and mesophilic aerobics in different fruits and vegetables, including tomatoes [54][55], citrus fruits [56][57], raspberry fruits [58], blueberries [59], strawberries [60], and ready-to-eat pomegranate arils [61]. The preharvest application of Aloe vera gel treatment was also previously tested and found to be effective in postharvest storage; specifically, it was found to reduce the decay incidence of table grapes [62][63].
In a different study [64], Aloe vera leaf gel was found to inhibit the growth of two bacteria: Shigella flexneri and Streptococcus progenes. The antibacterial activities of A. vera gel was also reported by Wang et al. [65] and Cellini et al. [66] against Heliobacter pylori. Moreover, antiviral activities of A. vera have also been of interest to many researchers, wherein its positive influence has been reported against herpes simplex virus (HSV) type 2 strains by Zandi and Rastian [67] and against influenza A virus replication by Li et al. [68].
References
- Cordenunsi, B.R.; Nascimento, J.R.O.; Lajolo, F.M. Physicochemical changes related to quality of five strawberry fruit cultivars during cool-storage. Food Chem. 2003, 83, 167–173.
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Cont. 2009, 50, 205–221.
- Coulibaly, O.; Nouhoheiflin, T.; Aitchedji, C.C.; Cherry, A.J.; Adegbola, P. Consumers’ Perceptions and Willingness to Pay for Organically Grown Vegetables. Int. J. Veg. Sci. 2011, 17, 349–362.
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf.-CRFSFS 2007, 6, 60–75.
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782.
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2015, 23, 872.
- Adiletta, G.; Pasquariello, M.S.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Petriccione, M. Chitosan coating: A postharvest treatment to delayoxidative stress in loquat fruits during cold storage. Agronomy 2018, 8, 54.
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Cont. 2015, 47, 381–391.
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256.
- Kahramanoğlu, İ.; Aktaş, M.; Gündüz, Ş. Effects of fludioxonil, propolis and black seed oil application on the postharvest quality of “Wonderful” pomegranate. PLoS ONE 2018, 13, e0198411.
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants 2019, 8, 26.
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 80–187.
- Dang, K.T.H.; Singh, Z.; Swinny, E.E. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J. Agric. Food Chem. 2008, 56, 1361–1370.
- Chen, C.; Peng, X.; Zeng, R.; Chen, M..; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48.
- Troyo, R.D.; Acedo, A.L. Effects of calcium ascorbate and calcium lactate on quality offresh-cut pineapple (Ananas comosus). Int. J. Agric. Life Sci. 2019, 3, 143–150.
- Misir, J.; Brishti, F.H.; Hoque, M.M. Aloe vera gel as a novel edible coating for fresh fruits: A Review. Am. J. Food Sci. Technol. 2014, 2, 93–97.
- Kahramanoğlu, İ. Introductory chapter: Postharvest physiology and technology of horticultural crops. In Postharvest Handling; Kahramanoğlu, İ., Ed.; InTech Open: London, UK, 2017; pp. 1–5.
- Singh, D.; Sharma, R.R. Postharvest diseases of fruits and vegetables and their management. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–52.
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B.E. Chemistry of Aloe Species. Curr. Org. Chem. 2000, 4, 1055–1078.
- Lv, L.; Yang, Q.Y.; Zhao, Y.; Yao, C.S.; Sun, Y.; Yang, E.J.; Fang, W.S. BACE1 (β-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med. 2008, 74, 540–545.
- Lee, S.; Do, S.G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass Spectrometry-Based Metabolite Profiling and Antioxidant Activity of Aloe vera (Aloe barbadensis Miller) in Different Growth Stages. J. Agric. Food Chem. 2012, 60, 11222–11228.
- Park, M.K.; Park, J.H.; Shin, Y.G.; Kim, W.Y.; Lee, J.H.; Kim, K.H. Neoaloesin A: A New C-Glucofuranosyl Chromone from Aloe barbadensis. Planta Medica 1996, 64, 363–365.
- Okamura, N.; Hine, N.; Tateyama, Y.; Nakazawa, M.; Fujioka, T.; Mihashi, K.; Yagi, A. Three chromones of Aloe vera leaves. Phytochemistry 1997, 45, 1511–1513.
- Okamura, N.; Hine, N.; Harada, S.; Fujioka, T.; Mihashi, K.; Yagi, A. Three chromone components from Aloe vera leaves. Phytochemistry 1996, 43, 495–498.
- Okamura, N.; Hine, N.; Tateyama, Y.; Nakazawa, M.; Fujioka, T.; Mihashi, K.; Yagi, A. Five chromones from Aloe vera leaves. Phytochemistry 1998, 49, 219–223.
- Okamura, N.; Hine, N.; Harada, S.; Fujioka, T.; Mihashi, K.; Nishi, M.; Miyahara, K.; Yagi, A. Diastereomeric C-glucosylanthrones of Aloe vera leaves. Phytochemistry 1997, 45, 1519–1522.
- Zhong, J.S.; Huang, Y.Y.; Zhang, T.H.; Liu, Y.P.; Ding, W.J.; Wu, X.F.; Xie, Z.Y.; Luo, H.B.; Wan, J.Z. Natural phosphodiesterase-4 inhibitors from the leaf skin of Aloe barbadensis Miller. Fitoterapia 2015, 100, 68–74.
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. Antiinflammatory C-Glucosyl Chromone from Aloe barbadensis. J. Nat. Prod. 1996, 59, 541–543.
- Kim, J.H.; Yoon, J.Y.; Yang, S.Y.; Choi, S.K.; Kwon, S.J.; Cho, I.S.; Jeong, M.H.; Kim, Y.H.; Choi, G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 78–83.
- Rehman, N.U.; Al-Riyami, S.A.; Hussain, H.; Ali, A.; Khan, A.L.; Al-Harrasi, A. Secondary metabolites from the resins of Aloe vera and Commiphora mukul mitigate lipid peroxidation. Acta Pharm. 2019, 69, 433–441.
- Rehman, N.U.; Hussain, H.; Khiat, M.; Khan, H.Y.; Abbas, G.; Green, I.R.; Al-Harrasi, A. Bioactive chemical constituents from the resin of Aloe vera. Z. Nat. Sect. B-A J. Chem. Sci. 2017, 72, 955–958.
- Rehman, N.U.; Hussain, H.; Khiat, M.; Al-Riyami, S.A.; Csuk, R.; Khan, H.Y.; Abbas, G.; Al-Thani, G.S.; Green, I.R.; Al-Harrasi, A. Aloeverasides A and B: Two Bioactive C-Glucosyl Chromones from Aloe vera Resin. Helv. Chim. Acta 2016, 99, 687–690.
- Yang, Q.Y.; Yao, C.S.; Fang, W.S. A new triglucosylated naphthalene glycoside from Aloe vera L. Fitoterapia 2010, 81, 59–62.
- Choi, J.S.; Lee, S.K.; Sung, C.K.; Jung, J.H. Phytochemical study on Aloe vera. Arch. Pharmacal Res. 1996, 19, 163–167.
- Tan, Z.J.; Li, F.F.; Xu, X.L. Extraction and purification of anthraquinones derivatives from Aloe vera L. using alcohol/salt aqueous two-phase system. Bioprocess Biosyst. Eng. 2013, 36, 1105–1113.
- Borges-Argaez, R.; Chan-Balan, R.; Cetina-Montejo, L.; Ayora-Talavera, G.; Sansores-Peraza, P.; Gomez-Carballo, J.; Caceres-Farfan, M. In vitro evaluation of anthraquinones from Aloe vera (Aloe barbadensis Miller) roots and several derivatives against strains of influenza virus. Ind. Crop. Prod. 2019, 132, 468–475.
- Epifano, F.; Fiorito, S.; Locatelli, M.; Taddeo, V.A.; Genovese, S. Screening for novel plant sources of prenyloxyanthraquinones: Senna alexandrina Mill. and Aloe vera (L.) Burm. F. Nat. Prod. Res. 2015, 29, 180–184.
- Lopez, A.; de Tangil, M.; Vega-Orellana, O.; Ramirez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954.
- Keyhanian, S.; Stahl-Biskup, E. Phenolic constituents in dried flowers of Aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007, 73, 599–602.
- Lawrence, R.; Tripathi, P.; Jeyakumar, E. Isolation, purification and evaluation of antibacterial agents from Aloe vera. Braz. J. Microbiol. 2009, 40, 906–915.
- Zhang, X.F.; Wang, H.M.; Song, Y.L.; Nie, L.H.; Wang, L.F.; Liu, B.; Shen, P.P.; Liu, Y. Isolation, structure elucidation, antioxidative and immunomodulatory properties of two novel dihydrocoumarins from Aloe vera. Bioorg. Med. Chem. Lett. 2016, 16, 949–953.
- Speranza, G.; Dadá, G.; Lunazzi, L.; Gramatica, P.; Manitto, P. Aloenin B, a New Diglucosylated 6-Phenyl-2-pyrone from Kenya Aloe. J. Nat. Prod. 1986, 49, 800–805.
- Tanaka, M.; Misawa, E.; Ito, Y.; Habara, N.; Nomaguchi, K.; Yamada, M.; Toida, T.; Hayasawa, H.; Takase, M.; Inagaki, M.; et al. Identification of five phytosterols from aloe vera gel as anti-diabetic compounds. Biol. Pharm. Bull. 2006, 29, 1418–1422.
- Afzal, M.; Ali, M.; Hassan, R.A.H.; Sweedan, N.; Dhami, M.S.I. Identification of Some Prostanoids in Aloe vera Extracts. Planta Med. 1991, 57, 38–40.
- Nabigol, A.; Asghari, A. Antifungal activity of Aloe vera gel on quality of minimally processed pomegranate arils. Int. J. Agron. Plant Prod. 2013, 4, 833–838.
- Kator, L.; Hosea, Z.Y.; Ene, O.P. The Efficacy of Aloe-vera coating on postharvest shelf life and quality tomato fruits during storage. Asian Res. J. Agric. 2018, 8, 1–9.
- Benitez, S.; Achaerandio, I.; Pujol, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in freshcut fruits: A case of study with kiwifruit slices. LWT-Food Sci. Technol. 2015, 61, 184–193.
- Krishnan, S.A.; Ullas, A.; Sagarika, N.; Oommen, T.E.; Sunaila, K. Development of Aloevera Based Edible Coating. Int. J. Pure App. Biosci. 2017, 5, 796–801.
- Sitara, U.; Hassan, N.; Naseem, J. Antifungal activity of Aloe vera gel against plant pathogenic fungi. Pak. J. Bot. 2011, 43, 2231–2233.
- Rosca-Casian, O.; Parvu, M.; Vlase, L.; Tamas, M. Antifungal activity of Aloe vera leaves. Fitoterapia 2007, 78, 219–222.
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocoll. 2017, 72, 1–10.
- Serrano, M.; Miguel, J.; Guillen, F.; Castillo, S.; Martinez-Romero, D.; Valero, D. Use of Aloe vera gel coating preserves the functional properties of table grapes. J. Agri. Food Chem. 2006, 54, 3882–3886.
- Navarro, D.; Díaz-Mula, H.M.; Guillén, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246.
- Garcia, M.A.; Ventosa, M.; Diazi, R.; Falco, S.; Casariego, A. Effects of Aloe vera coating on postharvest quality of tomato. Fruits 2014, 69, 117–126.
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016.
- Saks, Y.; Barkai-Golan, R. Aloe vera gel activity against plant pathogenic fungi. Postharvest Biol. Technol. 1995, 6, 159–165.
- Jhalegar, J.; Sharma, R.R.; Singh, D. Antifungal efficacy of botanicals against major postharvest pathogens of Kinnow mandarin and their use to maintain postharvest quality. Fruits 2014, 69, 223–237.
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT-Food Sci. Technol. 2015, 60, 495–501.
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan–Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97.
- Nasrin, T.A.A.; Rahman, M.A.; Hossain, M.A.; Islam, M.N.; Arfin, M.S. Postharvest quality response of strawberries with aloe vera coating during refrigerated storage. J. Hortic. Sci. Biotechnol. 2017.
- Martínez-Romero, D.; Castillo, S.; Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valeroa, D.; Serranoba, M. Aloe vera gel coating maintains quality and safety of ready-to-eatpomegranate arils. Postharvest Biol. Technol. 2013, 86, 107–112.
- Castillo, S.; Navarro, D.; Zapata, D.J.; Guillén, F.; Valero, D.; Martínez-Romero, D.; Serrano, M. Using Aloe vera as a preharvest treatment to maintain postharvest organic table grape quality. Acta Hortic. 2012, 933, 621–626.
- Chauhan, S.; Gupta, K.C.; Agrawal, M. Application of Biodegradable Aloe vera gel to control post-harvest decay and longer the shelf life of Grapes. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 632–642.
- Ferro, V.A.; Bradlbury, F.; Cameron, P.; Shakir, E.; Rahman, S.R.; Stimson, W.H. In vitro suscepitibilities of Shigella flexneri and Streptococcus pyogenes to inner gel of Aloe barbadensis Miller. Antimicrob. Agent Chemother. 2003, 47, 1137–1139.
- Wang, H.H.; Chung, J.G.; Ho, C.C.; Wu, L.T.; Chang, S.H. Aloe-emodin effects on arylamine N-Acetyltransferase activity in the bacterium heliobacter pylori. Planta Med. 1998, 64, 176–178.
- Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Genovese, S.; Locatelli, M.; Di Guilio, M. In vitro activity of Aloe vera inner gel against Heliobacter pylori strains. Lett. Appl. Microbiol. 2014, 59, 43–48.
- Zandi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773.
- Li, S.W.; Yang, T.C.; Lai, C.C.; Huang, S.H.; Liao, J.M.; Wan, L.; Lin, Y.J.; Lin, C.W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




