
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Huiru Zheng | + 2041 word(s) | 2041 | 2021-11-03 07:59:50 | | | |
| 2 | Lindsay Dong | + 1211 word(s) | 3252 | 2021-11-29 08:59:02 | | |
Video Upload Options
Aging refers to progressive physiological changes in a cell, an organ, or the whole body of an individual, over time. Aging-related diseases are highly prevalent and could impact an individual’s physical health. Recently, artificial intelligence (AI) methods have been used to predict aging-related diseases and issues, aiding clinical providers in decision-making based on patient’s medical records. Deep learning (DL), as one of the most recent generations of AI technologies, has embraced rapid progress in the early prediction and classification of aging-related issues.
1. Introduction
2. Deep Learning in Predicting Aging-Related Diseases
2.1. Age-related macular degeneration (AMD)
2.2. Cardiovascular and Respiratory Disorders in Aging People
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Pengbo Zhang and Fen Xu | The study analyses and explore the application value of DL for the prediction of possible complications of coronary heart disease, and its effect on improvement of nursing and care. | DL was applied to data of 182 patients (age from 48 to 80 years old, average age: (65.27 ± 7.34) years old), collected from health records, including their previous medical history, clinical diagnosis, examination results, abnormal indicators, living habits and other information. | High-risk patients with coronary heart disease indicate relation with old age, medical history, characteristics such as lack of cognition and unhealthy lifestyle. DL Application could effectively predict the risk of related complications of heart diseases in a more accurate way. |
The proposed model attained a high Accuracy of 87.5%. | [7] |
| Goallec et al. | Heart disease is one of the primary causes of death after age 65 and, with the world population aging. This study gain insights from DL models aiding in predicting heart age. | The study involved training of magnetic resonance videos MRI videos with 3D CNN, images with 2D CNN, time series ECG with 1D CNN over 45,000 heart MRI and electrocardiograms [ECG] from the UK Biobank within the range 45–81 years. | The study reported biomarkers, clinical phenotypes, diseases, environmental and socioeconomic biomarkers associated with accelerated heart aging. The study also highlighted the aorta, the mitral valve, and the interventricular septum as key anatomical features driving heart age prediction. | MRI-based anatomical features predicted age better than ECG-based electro-physiological features (RMSE = 2.89 ± 0.02 years vs. 6.09 ± 0.0.02 years). | [8] |
| Joyce D. Schroeder et al. | The study aims to predict Chronic obstructive pulmonary disease (COPD) using DL methods. | The study uses 6749 two-view chest radiograph exams (2012–2017) involving mean age as near to 60 years, also discussing COPD case of 62-year-old female. The frontal and lateral images are fed as inputs to two parallel convolutional neural networks (CNN) with pulmonary function tests (PFT) annotation. | A CNN Model trained on chest radiographs for quantitative prediction of COPD performs better than state-of-the-art algorithms of Natural Language Processing (NLP) in the field, attaining good accuracy. | AUC of 0.814 for prediction of obstructive lung disease. | [9] |
| Ju Gang Nam et al. | Detecting 10 common abnormalities (pneumothorax, mediastinal widening, pneumoperitoneum, nodule/mass, consolidation, pleural effusion, linear atelectasis, fibrosis, calcification and cardiomegaly) to evaluate its impact in predictive diagnostic and judging the timeliness of reporting. | The proposed approach used a ResNet34-based deep CNN over samples with mean ± SD age 57.6 ± 17.9 years on the chest radiographs. | The proposed model advanced the reporting time for critical and urgent cases, aiding better health in elderly people. | The study successfully detected 10 common abnormalities in two external validation datasets with high AUCs, ranging from 0.895 to 1.00. The training data of were curated by radiologists mostly without CT reference, intended to resemble radiologists’ performance, resulting in better results. | [10] |
| H. Suan-Chia Yang et al. | To predict a patient’s risk of developing lung cancer, using DL approaches | The analysis included 11,617 patients with lung cancer and 1,423,154 control patients with mean age 66 years. A total of 9261 cases of lung cancers were identified in subjects with age >= 55. CNNs have been applied to radiographic images of chest and to facilitate detection and low-dose computed tomography classification of pulmonary nodules in lungs. Xception architecture which includes a 126-layer CNN-based neural network with a moderate number of parameters, was used for feature extraction. | The study involved time-related sequential information from the medical histories to evaluate lung cancer risk in patients rather than relying on does not rely on smoking status, socioeconomic status, or BMI. | AUCs of 0.87 in patients with age ≥ 55. | [11] |
2.3. Aging People and Arthritis
The studies have shown that DL can be used effectively to prognosticate joint pain or arthritis outcomes. Such diseases could otherwise trigger inflammation that could lead to irreversible damage in aging people. The study in [12] demonstrated a comprehensive classification and regression analysis using a novel DL on rheumatoid arthritis to determine concrete numerical predictions of disease activity instead of just classifying high or low risk patients, henceforth making treating-to-(predicted)-target strategies better. It was observed that female patients face a higher risk of clinical progression in rheumatoid arthritis. Potentially, lifestyle, sleep or nutrition also contribute to disease prediction. The DL model developed serves as a potential tool for clinical decision support for patients suffering from rheumatoid arthritis. Leung et al. [13], predicted the risk of osteoarthritis and the likelihood of the patient undergoing the total knee replacement, using DL models. These models accurately predicted osteoarthritis progression in patients requiring a total knee replacement within a nine-year time span than traditional grading systems [13]. In this prognostic study [14], electronic health records were monitored for medications, patient demographics, laboratories visited, and of disease activity measures using DL models to prognosticate future patient outcomes for rheumatoid arthritis. This study forecasted RA disease activity for future clinic visits to better guide specialized treatment on an individualized basis. DL methods measured RA disease activity scores across two healthcare systems and suggested that the disease activity, laboratory values, and medications combined together are the strongest predictor of RA at every clinical visit. DL models trained on the large and diverse patient populations proved to be robust and provided useful insights for patient care.
2.4. Alzheimer’s Disease (AD): A Common Disease in Aging People
Alzheimer’s disease (AD) is a progressive brain disorder that gradually destroys brain memory, it is a common disease in aging people, which is caused by dementia. DL approaches have shown promising results for automated diagnosis and the multi-class classification of AD using resonance imaging and tomographic images. Table 2 highlights the review of studies applying DL over AD subjects. The accurate diagnosis of AD is important, especially at the disease’s early stages, so that patients undergo preventive measures even before the occurrence of irreversible brain damage. Deep Learning (DL) has become a common technique for the early diagnosis of AD. Brain imaging techniques are used to visualize the structure and function of the human brain. The most commonly used imaging technique of MRI helps in measuring brain volumes indicating any kind of degeneration due to AD. For the functional connectivity studies of the human brain, independent components analysis (ICA) has been widely used for analysing neuroimaging data [15]. In the study by Qiao et al. [16], a DL-based method was developed to distinguish AD from controls by fusing the functional connectivity. The study detected the underlying biomarkers of AD by analysing functional MRI. Intrinsic functional connectivity in AD patients was noted to be significantly reduced in subcortical brain regions of the hippocampus, amygdala, insula and putam [16].
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Qiao et al. | The study proposes DL classification framework with multivariate data-driven based feature extraction for automatic diagnosis of AD. | 34 participants with mean age 68.64 ± 9.85 years, were taken as sample from memory outpatient clinic at the Huashan Hospital of Fudan University. A total of 34 participants with mean age 65.55 ± 8.98 years, were invited by public advertisement to take part in the study. The proposed method was based on a three-level hierarchical partner matching independent component analysis (3LHPM-ICA) and Granger causality (GC) to determine effective connectivity features playing role in AD diagnosis. | Identified brain features that can serve as important biomarkers for AD. | Accuracy of 95.59% in diagnosing AD | [16] |
| Qureshi et al. | The study performed automatic assessment of dementia severity using a DL framework applied to resting-state functional magnetic resonance imaging (rs-fMRI) data. | The demographics included the participants with mean age of 73. The 3D-CNN-based DL classification framework is used in this study to assess dementia. | The research supported automatic classification of AD into two groups of disease severity (very mild and mild vs. moderate and severe) enabling important contributions for clinical practice. | Accuracy of >90% was achieved for the disease classification. | [17] |
| Choi and Jin et al. | The study aims to develop an automatic image interpretation system based on a deep convolutional neural network (CNN) to predict future cognitive decline in mild cognitive impairment (MCI) patients using flurodeoxyglucose and florbetapir positron emission tomography (PET) | Deep CNN was trained using 3-dimensional PET volumes of AD. The data used in this study included subjects recruited in Alzheimer’s Disease Neuroimaging Initiative-II (ADNI-2) with available baseline data on FDG and AV-45 PET (http://adni.loni.usc.edu, accessed on 17 October 2021) with a mean age of 73 years. |
Importance of DL as a practical tool for developing predictive neuroimaging biomarker. | Accuracy of 84.2% to predict cognitive decline in AD. | [18] |
| Ding et al. | A DL algorithm is used for an early prediction tool for Alzheimer’s disease providing therapeutic intervention by using biochemical and imaging tests. | PET imaging studies from 1002 patients, from Alzheimer’s Disease Neuroimaging Initiative (ADNI)-1, ADNI2, and ADNI-GO (Grand Opportunities) studies was considered. The average age of the patients was 76 years (range, 55–93 years) for men and 75 years (range, 55–96 years) for women. Convolutional neural network architecture, Inception v3, was used in this study stacking over 11 modules where each module consists of pooling layers and convolutional filters with rectified linear units as activation function over the two-dimensional images of 16 horizontal sections of the brain. |
A DL algorithm developed for successful early prediction of Alzheimer’s disease by using fluorine 18 fluorodeoxyglucose PET of the brain. | AUC of 98% for predicting AD. | [19] |
| Lu et al. | The study is aimed at identifying the stage of Alzheimer’s Disease (AD) patients through the use of mobility data and DL models. | The study applied a state-of-the-art architecture deep convolutional neural network, Inception-ResNet-V2, to pre-train brain images involving elderly people of age > 65. | Modelling to classify AD, showed outstanding characteristics as a progression biomarker. Interestingly, the study involved weighted brain structural image and information on participant sex. | Accuracy of AD classification found to be >90%. | [20] |
| Huang et al. | The research proposes a practical brain imaging-based AD diagnostic classifier using DL/transfer learning on MRI dataset of unprecedented size and diversity. | The data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The study chose 2861 T1-MR images, including AD subjects with a mean age of 76.13 ± 7.50. The study proposed a multi-modality CNN-based classifier for AD diagnosis and prognosis. | The work distinguishes between AD or potential AD patients from cognitively unimpaired (labelled as CN) subjects accurately and automatically using medical images to facilitate a fast-preclinical diagnosis. | AUC of 92.01% to differentiate between AD and Controls. | [21] |
2.5. Prediction over Spectrum of Age-Related Issues
The studies [22][23][24][25][26][27][28][29][30][31][32][33], highlighted DL-based prediction of other age-related other issues such as Type -2 diabetics, COVID-19 in older patients, coronary blockage in arteries, age-related eye diseases, brain age with old age, age-related disease gene associations, and heart stroke.
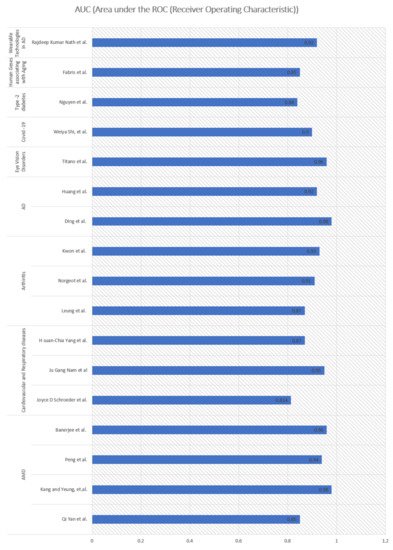
In general, overall high performance was achieved (>84% AUC) by adopting DL models in predicting aging-related diseases (Figure 1). Remarkable performance (high AUC of 98%) was achieved in studies involving DL [19] to predict AD in aging people.
3. Summary
-
DL learns the important patterns or relationships in large amounts of healthcare data and allows clinicians to perform model-based analysis integrated with their observations; leading to smart care achievable from such big data.
-
Remarkably, DL has achieved human-level performance in disease classification, learning over patterns/objects contained in medical images.
-
When DL is applied over the training data, it becomes more precise with multi-stream architecture and subsequently provides more accurate insights into care processes and diagnostics of aging diseases.
-
DL helps in the detection of clinically relevant features by learning patterns in medical imaging data beyond as perceived by a human observer/clinician.
-
DL approaches are now leading to lower costs and improved and faster outcomes in monitoring the health of aging people.
-
DL provides end-to-end learning models for heterogenous, uncertain and complex medical data.
-
DL provides clinicians with the support they need to understand medical environments.
References
- Rose, M.R.; Flatt, T.; Graves, J.L.; Greer, L.F.; Martinez, D.E.; Matos, M.; Mueller, L.D.; Shmookler Reis, R.J.; Shahrestani, P. What is aging? Front. Genet. 2012, 3, 134.
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187.
- Erin McNemar, M. Deep Learning, Predictive Analytics Helps Identify Chronic Diseases. Available online: https://healthitanalytics.com/news/deep-learning-predictive-analytics-helps-identify-chronic-diseases (accessed on 17 October 2021).
- Cao, C.; Liu, F.; Tan, H.; Song, D.; Shu, W.; Li, W.; Zhou, Y.; Bo, X.; Xie, Z. Deep Learning and Its Applications in Biomedicine. Genomics. Proteom. Bioinform. 2018, 16, 17–32.
- Yan, Q.; Weeks, D.E.; Xin, H.; Swaroop, A.; Chew, E.Y.; Huang, H.; Ding, Y.; Chen, W. Deep-learning-based prediction of late age-related macular degeneration progression. Nat. Mach. Intell. 2020, 2, 141–150.
- Yu-Chuan, E.; Yeung, L.; Lee, Y.-L.; Wu, C.-H.; Peng, S.-Y.; Chen, Y.-P.; Gao, Q.-Z.; Lin, C.; Kuo, C.-F.; Lai, C.-C. A Multimodal Imaging–Based Deep Learning Model for Detecting Treatment-Requiring Retinal Vascular Diseases: Model Development and Validation Study. JMIR Med Inf. 2021, 9, e28868.
- ZHANG, P.; XU, F. Effect of AI deep learning techniques on possible complications and clinical nursing quality of patients with coronary heart disease. Food Sci. Technol. 2021.
- Goallec, A.L.; Prost, J.-B.; Collin, S.; Diai, S.; Vincent, T.; Patel, C.J. Dissecting heart age using cardiac magnetic resonance videos, electrocardiograms, biobanks, and deep learning. medRxiv 2021.
- Schroeder, J.D.; Lanfredi, R.B.; Li, T.; Chan, J.; Vachet, C.; III, R.P.; Srikumar, V.; Tasdizen, T. Prediction of Obstructive Lung Disease from Chest Radiographs via Deep Learning Trained on Pulmonary Function Data. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 3455.
- Gang Nam, J.; Kim, M.; Park, J.; Jin Hwang, E.; Hyuk Lee, J.; Hee Hong, J.; Mo Goo, J.; Min Park, C. Development and validation of a deep learning algorithm detecting 10 common abnormalities on chest radiographs. Eur. Respir. J. 2021, 57, 2003061.
- Yang, H.-C.; Wang, Y.-H.; Bai, K.-J.; Wang, H.-H.; Li, Y.-C. Artificial Intelligence–Based Prediction of Lung Cancer Risk Using Nonimaging Electronic Medical Records: Deep Learning Approach. J. Med. Internet Res. 2021, 23, e26256.
- Kalweit, M.; Walker, U.A.; Finckh, A.; Müller, R.; Kalweit, G.; Scherer, A.; Boedecker, J.; Hügle, T. Personalized prediction of disease activity in patients with rheumatoid arthritis using an adaptive deep neural network. PLoS ONE 2021, 16, e0252289.
- Leung, K.; Zhang, B.; Tan, J.; Shen, Y.; Geras, K.J.; Babb, J.S.; Cho, K.; Chang, G.; Deniz, C.M. Prediction of Total Knee Replacement and Diagnosis of Osteoarthritis by Using Deep Learning on Knee Radiographs: Data from the Osteoarthritis Initiative. Radiology 2020, 296, 584–593.
- Norgeot, B.; Glicksberg, B.S.; Trupin, L.; Lituiev, D.; Gianfrancesco, M.; Oskotsky, B.; Schmajuk, G.; Yazdany, J.; Butte, A.J. Assessment of a Deep Learning Model Based on Electronic Health Record Data to Forecast Clinical Outcomes in Patients With Rheumatoid Arthritis. JAMA Netw. Open 2019, 2, e190606.
- Calhoun, V.D.; Liu, J.; Adalı, T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 2009, 45, S163–S172.
- Qiao, J.; Lv, Y.; Cao, C.; Wang, Z.; Li, A. Multivariate Deep Learning Classification of Alzheimer’s Disease Based on Hierarchical Partner Matching Independent Component Analysis. Front. Aging Neurosci. 2018, 10, 417.
- Qureshi, M.N.I.; Ryu, S.; Song, J.; Lee, K.H.; Lee, B. Evaluation of Functional Decline in Alzheimer’s Dementia Using 3D Deep Learning and Group ICA for rs-fMRI Measurements. Front. Aging Neurosci. 2019, 11, 8.
- Choi, H.; Jin, K.H. Predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behav. Brain Res. 2018, 344, 103–109.
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Aparici, C.M.; et al. A deep learning model to predict a diagnosis of Alzheimer disease by using 18 F-FDG PET of the brain. Radiology 2019, 290, 456–464.
- Lu, B.; Li, H.-X.; Chang, Z.-K.; Li, L.; Chen, N.-X.; Zhu, Z.-C.; Zhou, H.-X.; Li, X.-Y.; Wang, Y.-W.; Cui, S.-X.; et al. A Practical Alzheimer Disease Classifier via Brain Imaging-Based Deep Learning on 85,721 Samples. bioRxiv 2021.
- Huang, Y.; Xu, J.; Zhou, Y.; Tong, T.; Zhuang, X. Diagnosis of Alzheimer’s Disease via Multi-Modality 3D Convolutional Neural Network. Front. Neurosci. 2019, 13.
- Jonsson, B.A.; Bjornsdottir, G.; Thorgeirsson, T.E.; Ellingsen, L.M.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Stefansson, K.; Ulfarsson, M.O. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 2019, 10, 1–10.
- Nguyen, B.P.; Pham, H.N.; Tran, H.; Nghiem, N.; Nguyen, Q.H.; Do, T.T.; Tran, C.T.; Simpson, C.R. Predicting the onset of type 2 diabetes using wide and deep learning with electronic health records. Comput. Methods Programs Biomed. 2019, 182, 105055.
- Prince, J.; De Vos, M. A Deep Learning Framework for the Remote Detection of Parkinson’S Disease Using Smart-Phone Sensor Data. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3144–3147.
- Mishkhal, I.; Kareem, S.A.A.; Saleh, H.H.; Alqayyar, A. Deep Learning with network of Wearable sensors for preventing the Risk of Falls for Older People. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 032050.
- Nath, R.K.; Thapliyal, H.; Caban-Holt, A. Machine Learning Based Stress Monitoring in Older Adults Using Wearable Sensors and Cortisol as Stress Biomarker. J. Signal Process. Syst. 2021, 1–13.
- Titano, J.J.; Badgeley, M.; Schefflein, J.; Pain, M.; Su, A.; Cai, M.; Swinburne, N.; Zech, J.; Kim, J.; Bederson, J.; et al. Automated deep-neural-network surveillance of cranial images for acute neurologic events. Nat. Med. 2018, 24, 1337–1341.
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc. Imaging 2018, 11, 1654–1663.
- Shi, W.; Peng, X.; Liu, T.; Cheng, Z.; Lu, H.; Yang, S.; Zhang, J.; Wang, M.; Gao, Y.; Shi, Y.; et al. A deep learning-based quantitative computed tomography model for predicting the severity of COVID-19: A retrospective study of 196 patients. Ann. Transl. Med. 2021, 9, 216.
- Varadarajan, A.V.; Poplin, R.; Blumer, K.; Angermueller, C.; Ledsam, J.; Chopra, R.; Keane, P.A.; Corrado, G.S.; Peng, L.; Webster, D.R. Deep Learning for Predicting Refractive Error From Retinal Fundus Images. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2861–2868.
- Basheer, S.; Bhatia, S.; Sakri, S.B. Computational Modeling of Dementia Prediction Using Deep Neural Network: Analysis on OASIS Dataset. IEEE Access 2021, 9, 42449–42462.
- Cheon, S.; Kim, J.; Lim, J. The Use of Deep Learning to Predict Stroke Patient Mortality. Int. J. Environ. Res. Public Health 2019, 16, 1876.
- Papagiannaki, A.; Zacharaki, E.I.; Kalouris, G.; Kalogiannis, S.; Deltouzos, K.; Ellul, J.; Megalooikonomou, V. Recognizing Physical Activity of Older People from Wearable Sensors and Inconsistent Data. Sensors 2019, 19, 880.




