Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Osamu Hiraike | + 3334 word(s) | 3334 | 2021-11-10 02:20:49 | | | |
| 2 | Bruce Ren | Meta information modification | 3334 | 2021-11-17 09:00:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hiraike, O. Benefits of the Phytoestrogen Resveratrol for Perimenopausal Women. Encyclopedia. Available online: https://encyclopedia.pub/entry/16082 (accessed on 07 February 2026).
Hiraike O. Benefits of the Phytoestrogen Resveratrol for Perimenopausal Women. Encyclopedia. Available at: https://encyclopedia.pub/entry/16082. Accessed February 07, 2026.
Hiraike, Osamu. "Benefits of the Phytoestrogen Resveratrol for Perimenopausal Women" Encyclopedia, https://encyclopedia.pub/entry/16082 (accessed February 07, 2026).
Hiraike, O. (2021, November 17). Benefits of the Phytoestrogen Resveratrol for Perimenopausal Women. In Encyclopedia. https://encyclopedia.pub/entry/16082
Hiraike, Osamu. "Benefits of the Phytoestrogen Resveratrol for Perimenopausal Women." Encyclopedia. Web. 17 November, 2021.
Copy Citation
Endometriosis, characterized by macroscopic lesions in the ovaries, is a serious problem for women who desire conception. Damage to the ovarian cortex is inevitable when lesions are removed via surgery, which finally decreases the ovarian reserve, thereby accelerating the transition to the menopausal state. Resveratrol, a plant-derived molecule that promotes the function and expression of the sirtuin, SIRT1, has been attracting attention, and many reports have shown that resveratrol might exert cardiovascular protective effects.
dyslipidemia
hypertension
osteoporosis
endometriosis
resveratrol
sirtuin
1. Introduction
Endometriosis is defined as the growth of the extrauterine endometrial tissue in the presence of estrogen. Although many theories have been proposed to explain its pathophysiology, no definitive conclusion has yet been reached. The frequency of endometriosis is increasing, which is possibly because the frequency of conception in industrialized countries in recent years is lower than that in the early 1990s. In addition, women tend to hesitate to go to hospitals, although symptoms of endometriosis may appear early after menarche. Hence, endometriosis is generally diagnosed later in life, often after years of experiencing symptoms [1]. Unfortunately, the true prevalence of endometriosis in adolescent girls is unknown, and clinical symptoms and diagnostic challenges may arise when young girls are examined. This is mainly due to underestimation of the heavy burden for women who desire conception. Macroscopic lesions of endometriosis most frequently appear in the ovaries. Damage to the ovarian cortex is inevitable while surgically removing the lesions, and ovarian surgery ultimately decreases ovarian reserve, thereby accelerating the menopausal state [2]. Climacteric symptoms, osteopenia, and dyslipidemia occur soon after cessation of ovarian function [3]. Epidemiologically, there are sex-related differences in the frequency of dyslipidemia, hypertension, and osteoporosis. Females are more susceptible to these diseases than males, and their prevention is important for extending healthy life expectancy. In premenopausal women, the ovaries are the main source of estrogen. Menstruation is established at puberty, which indicates initiation of the ovulation cycle. Periodic changes in the secretion levels of estrogen, the main source of ovarian granulocyte cells, is observed with ovulation, and secretion of estrogen is generally maintained at high level. However, estrogen production diminishes with age, as the oocyte reserve is exhausted when women reach menopause. The secretion level of the follicle stimulating hormone, a pituitary hormone that promotes ovulation, increases significantly, while estrogen levels decrease significantly (Figure 1).
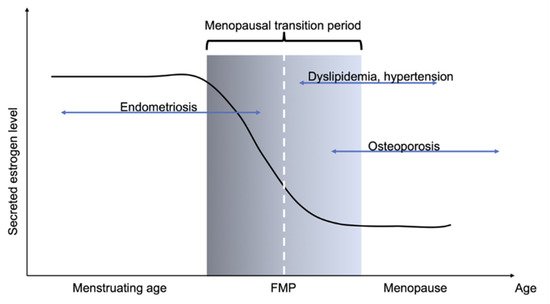
Figure 1. Schematic showing changes in estrogen level with age, and subsequent disease patterns. Menopausal transition period is generally considered between 45 and 55 years. An enigmatic disease, endometriosis is known to occur in women of reproductive age. The burden of endometriosis persists until menopause. Menopausal symptoms and vasomotor symptoms occur soon after cessation of menstruation. This is accompanied by worsening of dyslipidemia and hypertension. Decrease in estrogen level accelerates bone loss because of increase in bone resorption.
Postmenopausal estrogen level is generally low, and its secretion level is maintained by the presence of P450 aromatase located in peripheral adipose tissues, which converts testosterone into estrogen. Estrogen levels in postmenopausal women are lower than those in men in all generations. Collectively, women experience an inevitable decrease in ovarian function, and the presence of endometriosis accelerates the menopausal stage. It is believed that rapid decrease in estrogen is detrimental for the maintenance of women’s health, and that the decrease should be more modest. This theory is known as euestrogenemia [4]. In clinical practice, hormone replacement therapy cannot be prescribed to all women, as it should be optimized individually. Hence, instead using estrogen, safe administration of supplements to all women should be recommended for maintaining a healthy lifestyle.
Dyslipidemia and hypertension are associated with the progression of arteriosclerosis, and arteriosclerotic changes in the large and middle blood vessels are one of the main causes of myocardial and cerebral infarctions. Bone resorption is accelerated by activated osteoclasts, and rapid bone remodeling reduces bone mineral density. Osteoporosis is associated with aberrant fractures in the spine and hip, and the latter confines the patients to the bed. Resveratrol (3,5,40-trihydroxy-trans-stilbene), a plant-derived polyphenol that promotes the function and expression of SIRT1 (discussed later), has been attracting attention, and many reports have shown that resveratrol might exert cardiovascular protective effects. Preclinical reports also indicate that it can prevent bone loss and endometriosis.
2. Sirtuin Family Molecules and Resveratrol
2.1. Sirtuin Family Molecules and Their Functions
The silent information regulator 2 gene (Sir2) was first discovered in Saccharomyces cerevisiae as a possible longevity gene, the main function of which was identified to be a nicotinamide adenine dinucleotide (NAD+)-dependent ADP-ribosyltransferases. After the discovery of Sir2, orthologues of sirtuins (silent mating type information regulation 2 homolog) were reported, and seven human sirtuins have been identified (Table 1) [5].
Table 1. Sirtuin family molecules and their properties. Characteristics of human Sirtuins are described. Among them, SIRT1 and SIRT5 are postulated to be primary Sirtuins that respond to resveratrol. (a.a: amino acids, CPS-I: Carbamoyl phosphate synthase I RVT: resveratrol).
| Size (a.a) | Subcellular Localization |
Physiological Function |
Target | Physiological Functions |
Response to RVT | |
|---|---|---|---|---|---|---|
| SIRT1 | 746 | Nucleus | Deacetylation | PGC1α FOXO1 NFκB |
Metabolism Anti-inflammation Anti-Neurodegeneration |
↑ |
| SIRT2 | 388 | Cytosol | Deacetylation | H4 α-tublin |
Cell cycle Transcription |
ND |
| SIRT3 | 399 | Nucleus Mitochondria |
Deacetylation | AceCS2 | Metabolism | ↓ |
| SIRT4 | 314 | Mitochondria | ADP-ribosyl Transferase |
GDH | Insulin secretion | ND |
| SIRT5 | 310 | Mitochondria | Desuccinylase Deacetylation? |
Cytochrome C, CPS-I | Oxidative metabolism Apoptosis |
↑ |
| SIRT6 | 355 | Nucleus | ADP-ribosyl Transferase |
DNA polymerase β |
DNA repair | ND |
| SIRT7 | 400 | Nucleus | Unknown | RNA polymerase I |
Transcription of rDNA |
ND |
Phylogenetic analysis of sirtuin family molecules revealed that they can be divided into four subclasses: class I (SIRT1: Ia, SIRT2, and SIRT3: Ib), class II (SIRT4), class III (SIRT5), and class IV (SIRT6: IVa and SIRT7: IVb). They share a common catalytic domain as a unique protein structure and are primarily thought to act as NAD+-dependent histone deacetylases. Sirtuin enzymatic activity results in the formation of deacetylated lysine, nicotinamide, and O-acetyl-ADP-ribose. Among the sirtuin family molecules, SIRT1, SIRT3, and SIRT6 have been proposed to be involved in cardiovascular systems [6]. SIRT1 is primarily an NAD+-dependent deacetylase localized in the nucleus, and recent studies have indicated that SIRT1 can influence intracellular signaling by deacetylating factors such as p53, FOXO, PGC-1α, NF-κB, and β-catenin, thereby regulating gene expression, cell death, stress response, aging, and lipid and glucose metabolism (Figure 1) [7].
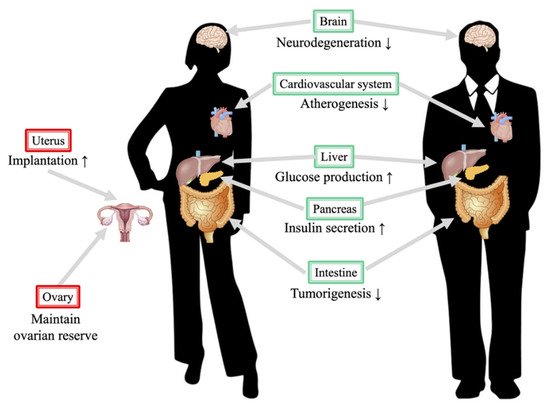
Figure 1. Protective roles of SIRT1 in the human body. The figure on the left denotes a female and that on the right denotes a male. Notably, several functions of SIRT1 in reproductive organs are beneficial. Resveratrol, a SIRT1 activator, promotes the luteinization function in the ovary [8], and several studies indicate that calorie restriction improves ovarian reserve [9]. Another beneficial mechanism is that SIRT1 might improve implantation in females via its effects on adhesion molecules [10].
SIRT1 also regulates autophagy flux by controlling FOXO1 and Rab7, which exerts a protective effect on cardiomyocytes [11]. Cardiac-specific overexpression of SIRT1 in a mouse model revealed that SIRT1 protects cardiac and renal functions affected by endoplasmic reticulum stress [12]. The regulatory mechanism of SIRT1 is complicated, as SIRT1 expression is upregulated during pressure overload. Calorie intake limitation, known as caloric restriction (CR), results in SIRT1 activation, and CR, exercise, and acute ischemic preconditioning stimulate SIRT1 expression. However, many hypotheses have been put forward to explain the mechanism underlying CR and SIRT1 activation; for example, CR promotes NAD+ production in cells, which suppresses the production of nicotinamide and promotes the expression of nicotinamide phosphoribosyltransferase and leads to adenosine monophosphate-activated protein kinase (AMPK) activation. CR is known to exert a protective effect against ischemic heart diseases. SIRT1 is known for its pleiotropic function, and we have demonstrated that SIRT1 can control the immune response of endometrial stromal cells by inhibiting TNFα-induced IL-6 secretion [13]. Taken together, SIRT1 appears to suppress the progression of endometriotic lesions.
In the sirtuin family, SIRT3 is primarily responsible for antioxidative stress effects and is known to exert a systemic effect, along with SIRT1. SIRT3 knockout mice exhibit systemic aging phenomena, such as deafness, myocardial hypertrophy, and blood vessel fibrosis [14]. Furthermore, it reportedly causes capillary abnormalities in the heart due to mitochondrial dysfunction. Enhancement of SIRT3 expression in cardiomyocytes possibly reduces the risk of heart failure, as it increases the expression of antioxidant factors, reverses fibrosis, and improves vascular endothelial cell function, thereby improving heart function [15]. Many studies have demonstrated the positive effects of SIRT3 on the cardiovascular system. In addition to its cardioprotective effect, recent studies have implicated the possible role of SIRT3 in maintaining bone metabolism, especially in bone resorption. Sirt3 deletion did not affect the skeleton of young mice; however, SIRT3 inhibited osteoclast differentiation, and deletion of SIRT3 increased trabecular bone mass in female mice. Another study also suggested the involvement of SIRT3 in maintaining healthy bone metabolism, as SIRT3 protects against advanced glycation end product-induced bone marrow mesenchymal stem cell (BMSC) senescence and contributes to improvement of bone mineral density. We speculated that SIRT3 activation might play dual roles in protecting bone metabolism, which varies with the age of the subjects. Although no report has suggested the role of SIRT3 in endometriosis, we have previously shown the role of SIRT3 in the ovaries. Significant oxidative stress appears after SIRT3 depletion in ovarian granulosa cells, which reduced the mRNA levels of aromatase, StAR, 17β-HSD, p450scc, and 3β-HSD [16].
As discussed, the sirtuin family has been found to be responsible for specific functions, which depend on the subtype. However, the mechanism of sirtuin stimulation by small-molecule compounds is poorly understood. A previous report using substrates and fluorophore-labeled peptides revealed a possible mechanism via which resveratrol inhibits human SIRT3. This was further confirmed using crystal structure analysis. The compound that activated SIRT3 acted as the top cover, and the binding pocket of the polypeptide was closed as a result of this binding, which enabled efficient and direct interaction with this substrate [17].
2.2. Function and Efficacy of Resveratrol in Cardiovascular Diseases and Osteoporosis
Resveratrol, a polyphenol present in red wine, can regulate vascular endothelial cells, pathological growth of smooth muscle cells, infiltration into the vascular walls of immune cells, and vascular remodeling [13][14]. Resveratrol might improve arterial stiffness and lower blood pressure in vivo. Resveratrol suppresses the expression of anti-apoptotic factors by increasing SIRT1 activation and expression both in vivo and in vitro. Resveratrol-induced SIRT1 activation not only increases the expression level of SIRT1 directly, but also enhances intracellular NAD+ concentration [18]. In addition, the activation of SIRT1 by resveratrol is also important for controlling autophagy, as autophagy flux positively acts on myocardial protection. In experiments using vascular smooth muscle cells (VSMCs), expression of the AT1 receptor is suppressed by the activation of SIRT1, which is induced by overexpression or resveratrol [19]. Resveratrol also suppresses cell death in cardiomyocytes via Ang II signaling, which is reported to increase superoxide dismutase (SOD) and SIRT1 expression levels [20]. In experiments using 24-month-old aging mice, AngII, prorenin receptor (PRR), and angiotensin converting enzyme (ACE) expression was suppressed by resveratrol administration [21]. Interestingly, the precise molecular mechanism via which resveratrol activates SIRT1 remains controversial [22]. High-throughput screening of chemicals that activate sirtuin revealed more than 14,000 compounds. SRT1720, SRT1460, SRT2183, and resveratrol were used in biochemical assays such as nuclear magnetic resonance, surface plasmon resonance, and isothermal calorimetry, in which they did not show any apparent activation with native peptide or full-length protein substrates [23]. Thus, the mechanism via which resveratrol activates SIRT1 is complicated, and the functions of resveratrol are now considered pleiotropic as they affect multiple receptors and substrates. A computational and biochemical analysis demonstrated that resveratrol can activate the deacetylase activity of SIRT3 and SIRT5, whereas resveratrol can inhibit the desuccinylase activity of SIRT5 [17]. This suggested that resveratrol can modulate sirtuins; thus, determining whether the effects of resveratrol are a central or peripheral issue in the human body is challenging.
In addition, resveratrol activates the transcription of ER as a phytoestrogen. Phytoestrogens can activate ER in the order of approximately 10−6~10−5 M in in vitro studies. Generally, phytoestrogens mainly act as ERα antagonist, but can weakly activate ERβ; therefore, phytoestrogens are considered to be SERM [24]. Resveratrol is known to possess polyphenolic properties; similar to catechins, resveratrol might possess antioxidative stress function and may activate nuclear factor-erythroid-derived-derived 2-related factor-2 (Nrf2), which is known to promote potent antioxidative stress effects. In a study using rat VSMCs, resveratrol promoted the expression of SIRT1 and Nrf2, resulting in the suppression of vascular calcification [25]. Nrf2 itself can be activated by resveratrol, and approximately 1 nM resveratrol is required for SIRT1 activation, indicating that the antioxidant effect of resveratrol depends on Nrf2. Thus, Nrf2 activation by dietary compounds is more realistic than SIRT1 activation, which requires micromolar levels of resveratrol. Nrf2 is a transcriptional factor that is degraded by Keap1 in the steady state. After exposure to oxidative stress, it translocates to the nucleus and binds to a specific lesion known as the antioxidant responsive element (ARE). After Nrf2 binds to ARE, downstream genes such as SOD, glutathione peroxidase (GPX), and heme oxygenase-1 (HO-1) are transcribed, resulting in a clear protective effect on blood vessels. Resveratrol activates SIRT1, ERβ, and Nrf2 concomitantly, and promotes the differentiation of many cell types; thus, resveratrol might have the potential to inhibit cell growth. A recent study indicated that resveratrol activates SIRT1 and SIRT5, while it has been suggested to suppress the function of SIRT3 using computational structural analysis. Resveratrol also contributes to the activation of AMPK [26]. Activation of AMPK has been shown to increase NO production [27] resulting in the activation of eNOS in murine models of high blood pressure [28]. In addition, PPARα is known to possess anti-inflammatory, anti-angiogenesis, and antioxidative stress properties. Resveratrol is known to activate PPARα, which results in activation of the AMPK pathway [29]. In a study using human umbilical vein endothelial cells (HUVECs), resveratrol suppressed HUVEC migration and monocyte chemotaxis by inhibiting monocyte chemoattractant protein-1 [30]. Resveratrol not only increased the expression of eNOS via AMPK, but also promoted the phosphorylation of eNOS.
Resveratrol has also been shown to exert beneficial effects on cultured cells, primary mesenchymal stem cells, preosteoblasts, and osteoclast progenitors in in vitro assays. Detailed reviews concluded that resveratrol might perform dual positive roles in bone, as it promotes osteoblastogenesis and inhibits osteoclastogenesis. Considering its dual positive role, treatment with resveratrol is ideal for maintaining bone mineral density in women at risk of osteoporosis. Previous studies have proposed various molecular mechanisms of resveratrol action. Cultured human BMSCs showed increased thymidine incorporation, maturation (as determined using alkaline phosphatase activity assay), and expression of osteoblastic markers (RUNX2/CBFA1, osterix, and osteocalcin). The effects were thought to be similar to those of 17β estradiol and were antagonized by ICI182,780, an ER antagonist. Resveratrol induced the activation of AMPK and suppressed the formation of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells in mouse bone marrow macrophage-derived osteoclasts. This mechanism was translated as negative regulation of RANKL by AMPK, and its function is related to Ca2+/calmodulin kinase (CaMK) and TGF-β-activated kinase-1 (TAK1). Effect of resveratrol on the Wnt signaling pathway has been investigated and resveratrol treatment of BMSCs dose-dependently enhanced β-catenin nuclear accumulation and positively regulated downstream TCF/LEF transcription, which induced osteoblastic cell differentiation; the increase in β-catenin accumulation was partially because resveratrol reduced the level of glycogen synthase kinase 3β. As explained above, resveratrol activated SIRT1 potently, which might play a prominent role in alleviating bone metabolism. One of the underlying mechanisms is that resveratrol promotes the interaction between SIRT1 and p300, a transcription coactivator, and represses RANK expression [31].
Low bioavailability is one of the major problems associated with resveratrol use. Although the concentration of resveratrol that can effectively activate SIRT1 in the experimental system is approximately in the micromolar range, this concentration could not be reached via oral administration. Topical administration may be an alternative approach. Reports show that stenosis of the rat carotid artery improved upon using stents containing resveratrol. If resveratrol is used for treating heart disease, we might expect prevention of arteriosclerosis, cardiac hypertrophy, and myocardial fibrosis. Currently, few reports have shown prevention of blood vessel calcification [25], and further investigation is necessary. As mentioned previously, resveratrol is a powerful natural antioxidant factor with potential for clinical application; in addition, other antioxidant substances might positively affect lipid metabolism. In future, we intend to use resveratrol to suppress cardiovascular events. Many reports have shown that resveratrol is a phytoestrogen; however, other phytoestrogens might also possess cardiovascular protective functions. Plants contain estrogen, such as isoflavones, stilbenes, flavones, and lignans. Among isoflavones, genistein, daidzein, and equol are used to alleviate symptoms of menopause. It is generally known that phytoestrogens can bind to ERα and ERβ and exhibit both agonistic and antagonistic actions against ER, although their binding capacity to ERβ is generally stronger than that to ERα. Therefore, phytoestrogens are considered to resemble SERM. Phytoestrogens regulate the proliferation of vascular endothelial cells, vascular permeability, NO production, and eNOS expression. It also suppresses the growth of VSMCs and inhibits AngII production.
2.3. Function and Efficacy of Resveratrol in Endometriosis
Resveratrol might improve the pathogenesis of endometriosis, and various mechanisms have been proposed using data derived from experimental preclinical studies. We have previously shown that SIRT1 can control the immune response of endometriotic stromal cells by inhibiting TNFα-induced IL-8 secretion [13], and that resveratrol can promote TNF-related apoptosis-inducing ligand-induced apoptosis in endometriotic stromal cells [32]. Studies using endometrial cancer cells also showed that resveratrol induced apoptosis but decreased cellular proliferation. Inhibition of COX-2 expression [33] and activation of AMPK pathway [34] are considered as possible mechanisms of action of resveratrol. Women with endometriosis possess poorer antioxidative stress ability, as evidenced by the decreased level of antioxidants in their peritoneal fluid compared to healthy women [35]. An experimental rodent model of endometriosis showed that resveratrol might play crucial roles in the resolution of endometriosis accompanied by improved antioxidative stress ability. Therefore, resveratrol is considered to prevent, treat, and cure endometriosis by attenuating reactive oxygen species. In addition, suppression of inflammatory cytokine secretion and modulation of M1/M2 macrophage polarization [36] may be another possible mechanism that explains the beneficial effects of resveratrol because increased inflammation is thought to induce oxidative stress in the peritoneal cavity [35]. Peritoneal invasion of endometrial tissues is suggested to play a crucial role in the inhibition of cellular invasion. Matrix metalloproteinases (MMPs) and their inhibitors known as tissue inhibitors of metalloproteinases (TIMPs) play fundamental roles in the establishment of peritoneal invasion of endometrial tissues and the ratio of MMPs/TIMPs is suggested to be important to determine the degree of peritoneal invasion [37]. Resveratrol may suppress the expression of MMP-2 and MMP-9 by enhancing SIRT1 expression, thus suppressing the invasion of endometrial tissues [38]. Sufficient blood supply is crucial for the invasion of endometrial tissues; thus, angiogenesis is regarded as an important factor to establish the implantation of ectopic endometrial tissues. Resveratrol could repress the angiogenic process by inhibiting both proangiogenic and angiogenic factors, which includes vascular endothelial growth factor and hypoxia-inducible factor 1α. Resveratrol could also inhibit capillary endothelial cell growth, thereby inhibiting new blood vessel formation [39].
Unfortunately, these observations are limited to in vitro or animal studies. Two clinical studies have utilized resveratrol as an adjunctive therapy for endometriosis. A study including 42 surgically confirmed patients with endometriosis prospectively received oral contraceptives (OCs) alone or together with resveratrol at the dose of 30 mg/body [40]. In another randomized clinical trial including 44 surgically confirmed patients with endometriosis, OCs with 40 mg/body resveratrol did not show any additive effect on dysmenorrhea-related pain relief [41]. The former research included women aged 22–37 years, and the treatment period of resveratrol was 2 months, while the latter included women aged 20–50 years, and the treatment period of resveratrol was 42 days. However, the study period for investigating the efficacy of resveratrol might be too short, and these studies used OCs and resveratrol concomitantly. Therefore, the beneficial role of resveratrol may still be ambiguous. Furthermore, these studies included patients with surgically confirmed endometriosis but the clinical details about the presence of endometrioma, deep infiltrating endometriosis, and superficial peritoneal lesions were not illustrated. We opted to use OCs for the treatment of endometriosis or primary dysmenorrhea in women aged less than 40 years. In fact, although it is not contraindicated, the World Health Organization recommends the use of OCs as medical eligibility category II for women aged more than 40 years [42]. Considering healthy aging, resveratrol may be used as an adjunctive therapy for maintaining cardiovascular systems and bone metabolism, and for preventing the development of endometriosis.
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256.
- Younis, J.S.; Shapso, N.; Ben-Sira, Y.; Nelson, S.M.; Izhaki, I. Endometrioma surgery—A systematic review and meta-analysis of the effect on antral follicle count and anti-Müllerian hormone. Am. J. Obstet. Gynecol. 2021.
- Dennerstein, L. A prospective population-based study of menopausal symptoms. Obstet. Gynecol. 2000, 96, 351–358.
- Turner, R.J.; Kerber, I.J. A theory of eu-estrogenemia: A unifying concept. Menopause 2017, 24, 1086–1097.
- Verdin, E.; Hirschey, M.; Finley, L.W.; Haigis, M.C. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010, 35, 669–675.
- Sosnowska, B.; Mazidi, M.; Penson, P.; Gluba-Brzózka, A.; Rysz, J.; Banach, M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis 2017, 265, 275–282.
- Finkel, T.; Deng, C.-X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591.
- Morita, Y.; Wada-Hiraike, O.; Yano, T.; Shirane, A.; Hirano, M.; Hiraike, H.; Koyama, S.; Oishi, H.; Yoshino, O.; Miyamoto, Y.; et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: An implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012, 10, 14.
- Xiang, Y.; Xu, J.; Li, L.; Lin, X.; Chen, X.; Zhang, X.; Fu, Y.; Luo, L. Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene 2012, 493, 77–82.
- Shirane, A.; Wada-Hiraike, O.; Tanikawa, M.; Seiki, T.; Hiraike, H.; Miyamoto, Y.; Sone, K.; Hirano, M.; Oishi, H.; Oda, K.; et al. Regulation of SIRT1 determines initial step of endometrial receptivity by controlling E-cadherin expression. Biochem. Biophys. Res. Commun. 2012, 424, 604–610.
- Ao, X.; Zou, L.; Wu, Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014, 21, 348–358.
- Huang, D.; Yan, M.-L.; Chen, K.-K.; Sun, R.; Dong, Z.-F.; Wu, P.-L.; Li, S.; Zhu, G.-S.; Ma, S.-X.; Pan, Y.-S.; et al. Cardiac-Specific Overexpression of Silent Information Regulator 1 Protects Against Heart and Kidney Deterioration in Cardiorenal Syndrome via Inhibition of Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2018, 46, 9–22.
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778.
- Kim, H.-S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell 2010, 17, 41–52.
- Wu, J.; Zeng, Z.; Zhang, W.; Deng, Z.; Wan, Y.; Zhang, Y.; An, S.; Huang, Q.; Chen, Z. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free. Radic. Res. 2019, 53, 139–149.
- Fu, H.; Wada-Hiraike, O.; Hirano, M.; Kawamura, Y.; Sakurabashi, A.; Shirane, A.; Morita, Y.; Isono, W.; Oishi, H.; Koga, K.; et al. SIRT3 Positively Regulates the Expression of Folliculogenesis- and Luteinization-Related Genes and Progesterone Secretion by Manipulating Oxidative Stress in Human Luteinized Granulosa Cells. Endocrinology 2014, 155, 3079–3087.
- Gertz, M.; Nguyen, G.T.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS ONE 2012, 7, e49761.
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646.
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a Longevity Gene, Downregulates Angiotensin II Type 1 Receptor Expression in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2008, 28, 1263–1269.
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of Manganese Superoxide Dismutase by Nuclear Translocation and Activation of SIRT1 Promotes Cell Survival in Chronic Heart Failure. J. Biol. Chem. 2010, 285, 8375–8382.
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis 2018, 270, 123–131.
- Moniot, S.; Weyand, M.; Steegborn, C. Structures, Substrates, and Regulators of Mammalian Sirtuins—Opportunities and Challenges for Drug Development. Front. Pharmacol. 2012, 3, 16.
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351.
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and β. Endocrinology 2000, 141, 3657–3667.
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386.
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433.
- Chen, Z.-P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, J.I.; Witters, L.A.; Power, D.A.; De Montellano, P.R.O.; Kemp, B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999, 443, 285–289.
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1723–1733.
- Okayasu, T.; Tomizawa, A.; Suzuki, K.; Manaka, K.-I.; Hattori, Y. PPARα activators upregulate eNOS activity and inhibit cytokine-induced NF-κB activation through AMP-activated protein kinase activation. Life Sci. 2008, 82, 884–891.
- Cicha, I.; Regler, M.; Urschel, K.; Goppelt-Struebe, M.; Daniel, W.G.; Garlichs, C.D. Resveratrol Inhibits Monocytic Cell Chemotaxis to MCP-1 and Prevents Spontaneous Endothelial Cell Migration Through Rho Kinase-Dependent Mechanism. J. Atheroscler. Thromb. 2011, 18, 1031–1042.
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 Interactions with p300 Modulate Receptor Activator of NF-κB Ligand (RANKL) Activation of NF-κB Signaling and Inhibit Osteoclastogenesis in Bone-derived Cells. J. Biol. Chem. 2011, 286, 11492–11505.
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol Enhances Apoptosis in Endometriotic Stromal Cells. Am. J. Reprod. Immunol. 2016, 75, 486–492.
- Sexton, E.; Van Themsche, C.; Leblanc, K.; Parent, S.; Lemoine, P.; Asselin, E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol. Cancer 2006, 5, 45.
- Fukuda, T.; Oda, K.; Wada-Hiraike, O.; Sone, K.; Inaba, K.; Ikeda, Y.; Makii, C.; Miyasaka, A.; Kashiyama, T.; Tanikawa, M.; et al. Autophagy inhibition augments resveratrol-induced apoptosis in Ishikawa endometrial cancer cells. Oncol. Lett. 2016, 12, 2560–2566.
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464.
- Laganà, A.S.; Salmeri, F.M.; Frangez, H.B.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2020, 36, 441–444.
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519.
- Lee, S.-J.; Kim, M.-M. Resveratrol with antioxidant activity inhibits matrix metalloproteinase via modulation of SIRT1 in human fibrosarcoma cells. Life Sci. 2011, 88, 465–472.
- Brâkenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800.
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Women’s Health 2012, 4, 543–549.
- DA Silva, D.M.; Gross, L.A.; Neto, E.D.P.G.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use Fifth Edition, 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/181468/9789241549158_eng.pdf (accessed on 30 October 2021).
More
Information
Subjects:
Reproductive Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
17 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




