
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bálint Lőrinczi | + 905 word(s) | 905 | 2021-11-09 10:41:44 | | | |
| 2 | Jason Zhu | Meta information modification | 905 | 2021-11-15 03:20:17 | | |
Video Upload Options
Kynurenic acid (KYNA) is an endogenous neuroprotective agent of increasing importance. Several derivatives have already been synthesized, bearing an abundance of functional groups attached to the main skeleton in different positions. Several of these compounds have already been tested in biological evaluations, with several of them targeting the same receptors and biological effects as KYNA.
1. Introduction
Among the important features of KYNA, it is one of few known endogenous excitatory amino acid receptor blockers with a broad spectrum of antagonistic properties in supraphysiological concentrations. It is well established that KYNA has a high affinity towards N-methyl-D-aspartate (NMDA) receptors. Moreover, it has recently been disclosed that KYNA shows an even higher affinity towards positive modulatory binding sites at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor [1].
Since KYNA is a neuroprotective agent able to prevent neuronal loss following excitotoxic, ischemia-induced, and infectious neuronal injuries [2][3], there has recently been increasing interest in the synthesis and pharmacological studies of KYNA derivatives. The substitution of KYNA at positions 5–8 was achieved by starting from the corresponding aniline via the modified Conrad–Limpach method [4][5][6]. The hydroxy group at position four was transformed to ether [6][7][8] or amine functions [9][10][11], while the carboxylic function at position two was mostly modified into the corresponding esters [6][7][8] or amides [12][13][14][15][16][17].
2. Mannich-Type Transformations of KYNA and Its Substituted Derivatives
2.1. C-3 Substitutions of KYNA Derivatives
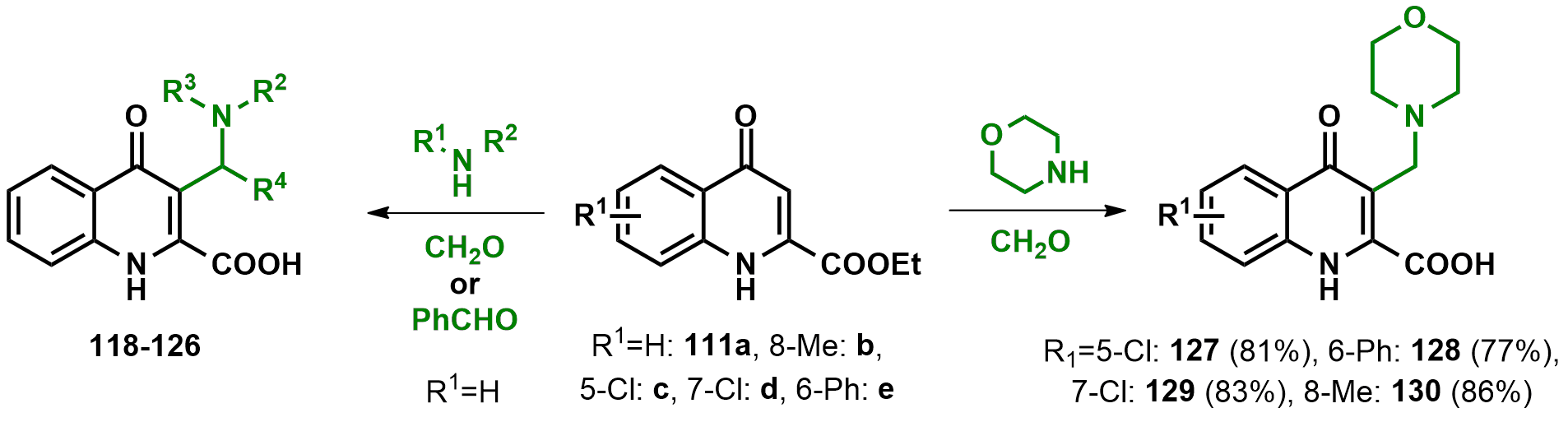
| Compound # | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|
| 118 | H | 2-morpholinoethyl | H | 78 |
| 119 | H | 2-(dimethylamino)ethyl | Ph | 74 |
| 120 | Me | Me | H | 82 |
| 121 | Me | Bz | H | 85 |
| 122 | morpholine | H | 91 | |
| 123 | piperidine | H | 73 | |
| 124 | N-methyl-piperazine | H | 78 | |
| 125 | 1,2,3,4-tetrahydroisoquinoline | H | 81 | |
| 126 | 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline | H | 72 | |
2.2. Aminoalkylations of Hydroxy-KYNA Derivatives
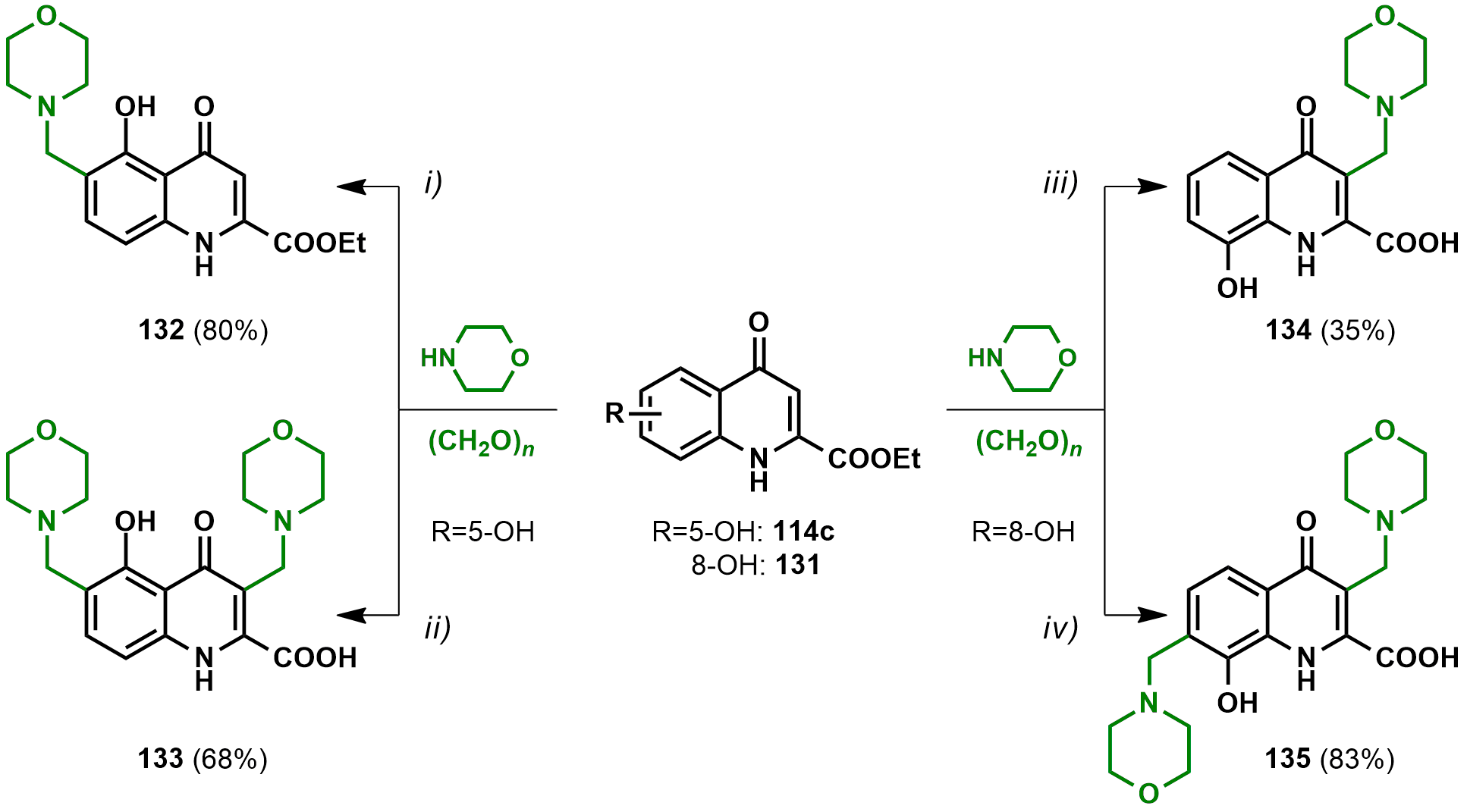
2.2.1. Derivatives with Structural Similarities to 1-Napthol
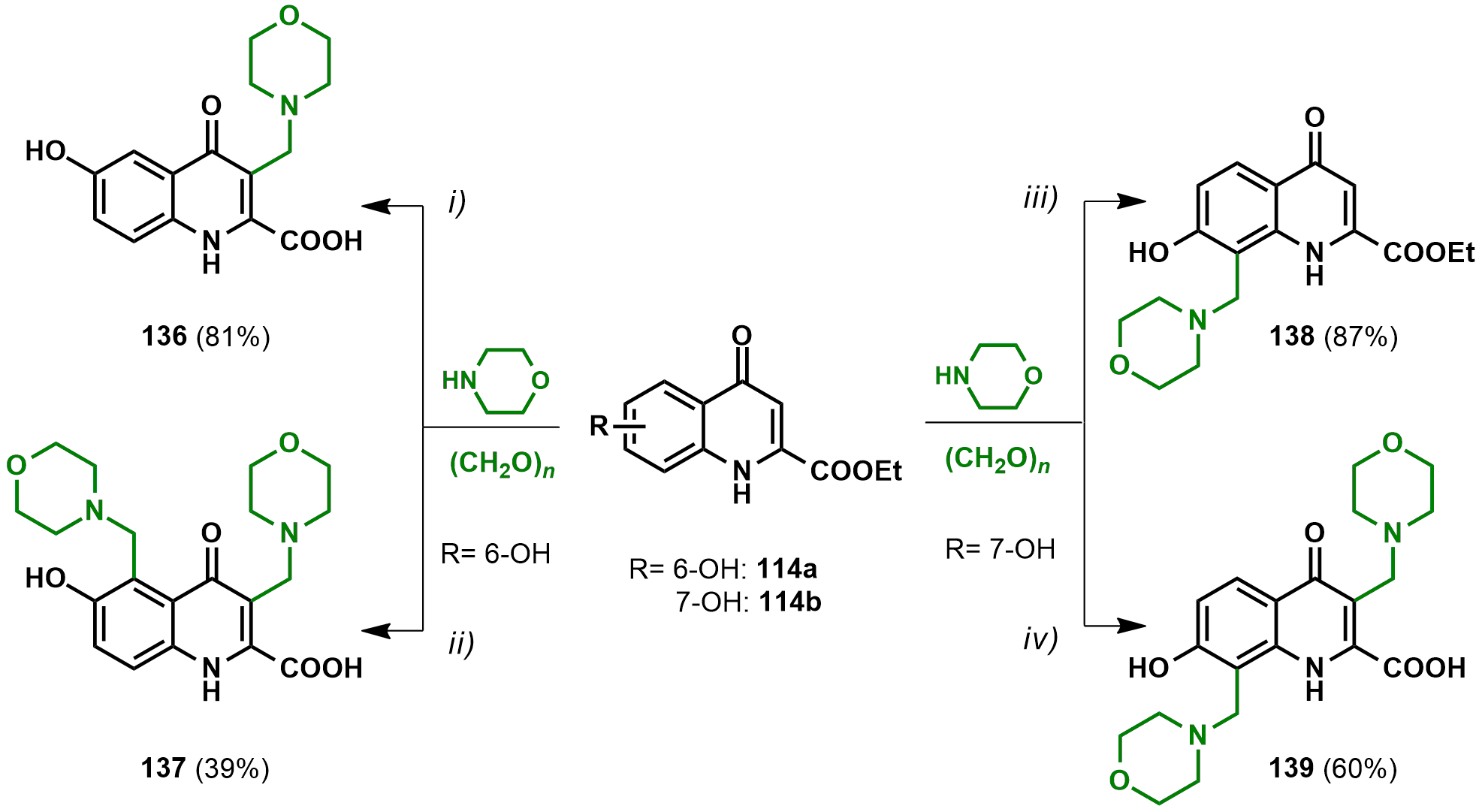
2.2.2. Derivatives with Structural Similarities to 2-Napthol
References
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007, 257, 221–239.
- Gigler, G.; Szénási, G.; Simó, A.; Lévay, G.; Hársing, L.G., Jr.; Sas, K.; Vécsei, L.; Toldi, J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007, 564, 116–122.
- Luchowska, E.; Luchowski, P.; Sarnowska, A.; Wielosz, M.; Turski, W.A.; Urbańska, E.M. Endogenous level of kynurenic acid and activities of kynurenine aminotransferases following transient global ischemia in the gerbil hippocampus. Pol. J. Pharmacol. 2003, 55, 443–447.
- Harrison, B.L.; Baron, B.M.; Cousino, D.M.; McDonald, I.A. 4-- and 4--5,7-dichloroquinoline-2-carboxylic acid: New antagonists of the strychnine-insensitive glycine binding site on the N-methyl-D-aspartate (NMDA) receptor complex. J. Med. Chem. 1990, 33, 3130–3132.
- Edmont, D.; Rocher, R.; Plisson, C.; Chenault, J. Synthesis and evaluation of quinoline carboxyguanidines as antidiabetic agents. Bioorg. Med. Chem. Lett. 2000, 16, 1831–1834.
- Bonina, F.P.; Arenare, L.; Ippolito, R.; Boatto, G.; Battaglia, G.; Bruno, V.; De Caprariis, P. Synthesis, pharmacokinetics and anticonvulsant activity of 7-chlorokynurenic acid prodrugs. Int. J. Pharm. 2000, 202, 79–88.
- Manfredini, S.; Pavan, B.; Vertuani, S.; Scaglianti, M.; Compagnone, D.; Biondi, C.; Scatturin, A.; Tanganelli, S.; Ferraro, L.; Prasad, P.; et al. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J. Med. Chem. 2002, 45, 559–562.
- Manfredini, S.; Vertuani, S.; Pavan, B.; Vitali, F.; Scaglianti, M.; Bortolotti, F.; Biondi, C.; Scatturin, A.; Prasad, P.; Dalpiaz, A. Design, synthesis and in vitro evaluation on HRPE cells of ascorbic and 6-bromoascorbic acid conjugates with neuroactive molecules. Bioorgan. Med. Chem. 2004, 12, 5453–5463.
- Yielding, K.L.; Nichols, A.C. Anticonvulsant activity of antagonists for the NMDA-associated glycine binding site. Mol. Chem. Neuropathol. 1993, 19, 269–282.
- Stone, T.W. Inhibitors of the kynurenine pathway. Eur. J. Med. Chem. 2000, 35, 179–186.
- Nichols, A.C.; Yielding, K.L. Anticonvulsant activity of 4-urea-5,7-dichlorokynurenic acid derivatives that are antagonists at the NMDA-associated glycine binding site. Mol. Chem. Neuropathol. 1998, 35, 1–12.
- Füvesi, J.; Somlai, C.; Németh, H.; Varga, H.; Kis, Z.; Farkas, T.; Károly, N.; Dobszay, M.; Penke, Z.; Penke, B.; et al. Comparative study on the effects of kynurenic acid and glucosamine-kynurenic acid. Pharmacol. Biochem. Behav. 2004, 77, 95–102.
- Zhang, L.; Sun, F.; Li, Y.; Sun, X.; Liu, X.; Huang, Y.; Zhang, L.H.; Ye, X.S.; Xiao, J. Rapid Synthesis of Iminosugar Derivatives for Cell-Based In Situ Screening: Discovery of “Hit” Compounds with Anticancer Activity. ChemMedChem 2007, 2, 1594–1597.
- Brik, A.; Lin, Y.C.; Elder, J.; Wong, C.H. A quick diversity-oriented amide forming reaction coupled with in situ screening as an approach to identify optimal p-subsite residues of HIV-1 protease inhibitors. Chem. Biol. 2002, 9, 891–896.
- Tossi, A.; Benedetti, F.; Norbedo, S.; Skrbec, D.; Berti, F.; Romeo, D. Small hydroxyethylene-based peptidomimetics inhibiting both HIV-1 and C. albicans aspartic proteases. Bioorg. Med. Chem. 2003, 11, 4719–4727.
- Knyihár-Csillik, E.; Mihály, A.; Krisztin-Péva, B.; Robotka, H.; Szatmári, I.; Fülöp, F.; Toldi, J.; Csillik, B.; Vécsei, L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: Comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008, 61, 429–432.
- Fülöp, F.; Szatmári, I.; Vámos, E.; Zádori, D.; Toldi, J.; Vécsei, L. Syntheses, transformations and pharmaceutical applications of kynurenic acid derivatives. Curr. Med. Chem. 2009, 16, 4828–4842.
- Szatmári, I.; Fülöp, F. Syntheses, transformations and applications of aminonaphthol derivatives prepared via modified Mannich reactions. Tetrahedron 2013, 69, 1255–1278.
- Szatmári, I.; Fülöp, F. Microwave-Assisted One-Pot Synthesis of (Aminoalkyl)naphthols and (Aminoalkyl)quinolinols by Using Ammonium Carbamate or Ammonium Hydrogen Carbonate as Solid Ammonia Source. Synthesis 2009, 5, 775–778.
- Szatmári, I.; Fülöp, F. Simple access to pentacyclic oxazinoisoquinolines via an unexpected transformation of aminomethylnaphthols. Tetrahedron Lett. 2011, 52, 4440–4442.
- Sas, J.; Szatmári, I.; Fülöp, F. C-3 functionalization of indole derivatives with isoquinolines. Curr. Org. Chem. 2016, 20, 2038–2054.
- Lőrinczi, B.; Csámpai, A.; Fülöp, F.; Szatmári, I. Synthesis of new C-3 substituted kynurenic acid derivatives. Molecules 2020, 25, 937.
- Lőrinczi, B.; Csámpai, A.; Fülöp, F.; Szatmári, I. Synthetic-and DFT modelling studies on regioselective modified Mannich reactions of hydroxy-KYNA derivatives. RSC Adv. 2021, 11, 543.




