Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mina S Makary | + 1481 word(s) | 1481 | 2021-11-04 03:40:35 | | | |
| 2 | Conner Chen | Meta information modification | 1481 | 2021-11-10 06:51:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Makary, M. Portal Vein Thrombosis in Hepatocellular Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/15739 (accessed on 08 February 2026).
Makary M. Portal Vein Thrombosis in Hepatocellular Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/15739. Accessed February 08, 2026.
Makary, Mina. "Portal Vein Thrombosis in Hepatocellular Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/15739 (accessed February 08, 2026).
Makary, M. (2021, November 05). Portal Vein Thrombosis in Hepatocellular Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/15739
Makary, Mina. "Portal Vein Thrombosis in Hepatocellular Carcinoma." Encyclopedia. Web. 05 November, 2021.
Copy Citation
Hepatocellular carcinoma is the fourth leading cause of cancer worldwide, and the fastest increasing cause of cancer mortality in the United States. Its propensity for vascular invasion leads to the presence of portal vein tumor thrombus in up to half of patients.
HCC
portal vein tumor thrombus
1. Hepatocellular Carcinoma
Hepatocellular cancer is the fourth leading cause of cancer deaths. The incidence and mortality are highest in East Asia and Africa, but HCC is the fastest increasing cause of cancer mortality in the US [1]. The strongest risk factor for HCC is cirrhosis, present in 80–90% of HCC cases, and HCC is the leading cause of death in cirrhotic patients. Most commonly, HCC develops from cirrhosis due to hepatitis B (HBV) or C infection, alcohol consumption, or diabetes or obesity-related NASH. The etiology of HCC has considerable geographic variation: in Asia and Africa, HCC secondary to HBV accounts for roughly 60% of cases, but only 20% in the United States and Europe [1]. Given that chronic hepatitis B and cirrhosis are well-established risk factors for HCC, screening ultrasound with or without AFP is recommended every 6 months in these groups and has been associated with a 37% decrease in HCC mortality [2]. Definitive diagnosis is made with a triple phase CT demonstrating the presence of lesions with arterial enhancement and delayed washout, a finding that has a sensitivity of 89% and a specificity of 96% for HCC [1].
Despite screening recommendations for patients with cirrhosis or hepatitis B, many patients are diagnosed with advanced disease after developing abdominal pain or weight loss. Prognosis and therapy depend on tumor stage, which is defined by the Barcelona Clinic Liver Cancer (BCLC) in the United State and Europe. This staging system combines three factors: disease extension, liver function status as defined by the Child–Pugh score, and Eastern Cooperative Oncology Group (ECOG) performance status (Figure 1). Very early stage HCC, or BCLC 0, is defined as a single nodule less than 2 cm, a Child–Pugh score of A and an ECOG score of 0. Early stage HCC, or BCLC A, is defined as one to three nodules less than 3 cm, a Child–Pugh score of A or B, and an ECOG score of 0. Intermediate stage HCC, or BCLC B, is defined as multinodular HCC, and the functional and performance criteria are unchanged. Advanced stage HCC, or BCLC C, is defined as the presence of portal invasion, regional nodal metastasis, or distant metastasis, a Child–Pugh of A or B, and an ECOG of 1–2. Finally, terminal stage HCC, or BCLC D, is defined as Child–Pugh C or ECOG > 2 [3].
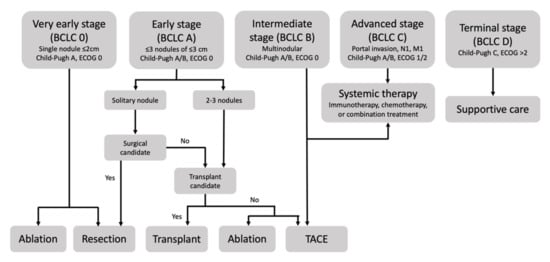
Figure 1. The Barcelona Clinic Liver Classification defines five stages of hepatocellular carcinoma based on assessment of disease distribution, liver function, and performance status and recommends therapy based on stage.
According to the BCLC, early stages are treated with resection, ablation, or transplant, intermediate stages are treated with transarterial chemoembolization, and advanced stages are treated with systemic chemotherapy. For early, intermediate, and advanced stages, survival is approximately 36, 16, and 6 months, respectively, in patients with preserved liver function (Child–Pugh A) [1]. However, recent advances in locoregional therapies including transarterial chemoembolization (TACE), transarterial radioembolization (TARE), ablation, radiotherapy, and hepatic intra-arterial infusion chemotherapy (HAIC) have demonstrated the potential to improve overall survival beyond six months in patients with advanced HCC complicated by PVTT.
2. Portal Vein Thrombosis
The liver receives a dual blood supply from the portal vein and the hepatic arteries. The portal vein is the primary blood supply to the liver, providing an estimated two-thirds of blood flow in the normal case. In a healthy, non-cirrhotic liver, the potential ischemic effects of portal vein thrombosis are mitigated by two compensatory mechanisms: dilation of the hepatic arteries and the rapid development in a matter of days of a system of venous collaterals adjacent to the portal vein, termed cavernous transformation. To maintain perfusion through this collateral system, portal pressures increase, which increases the risk of developing varices and associated bleeding [4]. In cirrhotic patients, the pathophysiology of portal vein thrombosis is related to the increased resistance to flow in the hepatic parenchyma secondary to fibrosis. This in turn slows portal flow rates, increasing the propensity for thrombus formation [5]. Additionally, like many cancers, HCC promotes a hypercoagulable state and also has a propensity for direct vascular invasion of the portal vein. The presentation of portal vein thrombosis is variable and may include abdominal pain, nausea, vomiting, and diarrhea in partial occlusion. In complete occlusion, it may present with abdominal pain and associated decompensation of chronic liver disease including ascites and variceal bleeding. Diagnosis of portal vein thrombosis is made with either Doppler ultrasound or contrast-enhanced CT, and can be can be classified as acute, subacute, or chronic. Treatment with anticoagulation is first line, and intervention can be considered in refractory symptomatic patients or those with complications from variceal bleeding.
HCC has a propensity for vascular invasion, particularly of the portal vein, resulting in a specific case of portal vein occlusion termed portal vein tumor thrombosis (PVTT), which does not respond to anticoagulation. PVTT affects between 35–50% of patients with HCC [6] and the presence of PVTT designates advanced disease (BCLC stage C). The poor prognosis is due to the risk of intravascular metastatic spread, increased risk of complications such as bleeding esophageal varices [7], and impaired liver function due to occlusion of the liver’s primary blood supply and worsening portal hypertension. For patients with PVTT, transplant is contraindicated, and the National Cancer Comprehensive Network (NCCN) recommendation for advanced HCC is locoregional therapy or systemic treatment. First line systemic treatment is with atezolizumab plus bevacizumab [8]. While sorafenib has long been the standard of care for these patients based on the results of two randomized controlled trials [9][10], combination atezolizumab plus bevacizumab recently demonstrated superiority in a landmark trial [11]. In this trial, combination therapy was associated with a hazard ratio for death of 0.58 compared to sorafenib (p < 0.001), improved overall survival at 12 months (67.2% vs. 54.6%), and improved progression-free survival (6.8 vs. 4.3 months). The NCCN guidelines acknowledge that there is currently insufficient evidence to recommend systemic therapy over locoregional therapy [8], and recent studies of locoregional therapies such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE) and ablative therapies have demonstrated benefit in HCC with PVTT in select cases.
3. Portal Vein Tumor Thrombus Classification
The portal vein is formed from the confluence of the superior mesenteric vein and the splenic vein, and within the liver, it divides into a left and right branch. Various further subdivisions supply the hepatic sinusoids. Thus, portal vein thrombus may involve distal branches of the portal vein and affect only a segment or a lobe, or it may involve the main trunk of the portal vein, affecting the entire liver.
In order to understand the literature regarding HCC with PVTT, it is important to be aware of the various classification systems commonly used—The Liver Cancer Study Group of Japan, the Cheng Classification, and the Xu classification—as the location of PVTT impacts prognosis and therapeutic options (Figure 2). The Vp classification system from the Liver Cancer Study Group of Japan is the most commonly used [12]. In this system, Vp0 indicates no tumor in the portal vein, Vp1 indicates tumor distal to second order branches, Vp2 indicates tumor within second order branches, Vp3 indicates tumor in first order branches, and Vp4 indicates tumor in the main portal vein. This must be carefully distinguished from the Cheng classification, where Type 0 indicates PVTT seen only with microscopy, Type 1 indicates PVTT in the second-order segmental branches, Type 2 indicates PVTT in the right or left portal vein, Type 3 indicates PVTT in the main portal vein, and Type 4 indicates PVTT in the superior mesenteric vein [13]. Finally, the Xu classification system specifies Type A as involvement of the main portal vein or both the right and left portal veins and Type B as involvement of either the right or left portal vein [14].
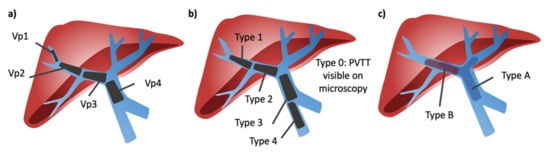
Figure 2. Portal vein tumor thrombosis classification systems. (a) Liver Cancer Study Group of Japan classification; Vp1 = thrombus located beyond second order branches, Vp2 = thrombus located in the second order branches, Vp3 = thrombus located in the first order branches, Vp4 = thrombus located in the main portal vein. (b) Cheng classification; Type 0 = PVTT seen only with microscopy, Type 1 = PVTT in the second-order segmental branches, Type 2 = PVTT in the right or left portal vein, Type 3 = PVTT in the main portal vein, and Type 4 = PVTT in the superior mesenteric vein. (c) Xu classification; type A = thrombus in main portal vein or both right and left portal veins, type B = thrombus in either right or left portal vein.
The need for a classification system arose in China and Japan as a result of guidelines that permit resection as a management strategy for HCC with PVTT. In literature from the United States and Europe, where these classification systems are less common, ambiguity arises when terms like segmental or sub-segmental PVTT are used to group PVTT patients. Here, segmental is used to mean any portal vein tumor thrombus outside of the main portal vein.
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–28.
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422.
- Llovet, J.M.; Fuster, J.; Bruix, J. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant. 2004, 10, S115–S120.
- Valla, D.C.; Condat, B. Portal vein thrombosis in adults: Pathophysiology, pathogenesis and management. J. Hepatol. 2000, 32, 865–871.
- Mantaka, A.; Augoustaki, A.; Kouroumalis, E.A.; Samonakis, D.N. Portal vein thrombosis in cirrhosis: Diagnosis, natural history, and therapeutic challenges. Ann. Gastroenterol. 2018, 31, 315.
- Cerrito, L.; Annicchiarico, B.E.; Iezzi, R.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J. Gastroenterol. 2019, 25, 4360.
- Lim, J.; Kim, H.I.; Kim, E.; Kim, J.; An, J.; Chang, S.; Kim, S.O.; Lee, H.C.; Lee, Y.S.; Shim, J.H. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: A matched nested case-control study. BMC Cancer 2021, 21, 1–10.
- National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 28 October 2021).
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Ikai, I.; Kudo, M.; Arii, S.; Omata, M.; Kojiro, M.; Sakamoto, M.; Takayasu, K.; Hayashi, N.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2010, 40, 1043–1059.
- Shi, J.; Lai, E.C.H.; Li, N.; Guo, W.X.; Xue, J.; Lau, W.Y.; Wu, M.C.; Cheng, S.Q. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 74–80.
- Xu, J.; Liu, X.; Wang, S.; Wen, H. Surgical treatment for hepatocellular carcinoma with portal vein tumor thrombus: A novel classification. World J. Surg. Oncol. 2015, 13, 86.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
10 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




