
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodney Hull | + 2266 word(s) | 2266 | 2021-10-19 10:29:28 | | | |
| 2 | Camila Xu | Meta information modification | 2266 | 2021-10-27 03:04:39 | | |
Video Upload Options
The microbiota–gut–brain axis consists of the brain, glands, gut, immune cells, and gastrointestinal microbiota. Both the central and enteric nervous systems regulate the communication between the gastrointestinal tract and the brain and apart from the nervous system, it is also regulated through hormones and immunological signalling. Multiple lines of evidence confirm the existence of the gut–brain axis.
1. Introduction
It has been estimated that the microbiota in a human body consists of approximately 3000 different species of microbe with 40,000,000,000,000 (40 trillion) individual microbial cells. The vast majority of these are gastrointestinal bacteria [1]. One of the first microbial based treatments proposed for cancer was found in the Eber’s Papyrus, written by the Egyptian physician Imhotep around the year 2600 BCE [2]. He proposed that infection of the area affected by the cancer through an open wound covered by a poultice could have therapeutic potential. At that time Germ theory was unknown [3]. This treatment was revived in the 1800s by William Coley who “vaccinated” cancer patients with live or heat-killed Streptococcus and Serratia species [2]. Coley documented 4 cases of sarcoma where the patients were completely cured by erysipelas infection. The medical establishment, however, discounted his treatment as fatal. We now know, erysipelas causes an inflammatory reaction that most probably is negating the sarcoma [4][5]. Coley’s infections with erysipelas showed for the first time that microbes influence the development and progression of cancer. The use of microbe metabolites, supplementing the diets with probiotics or by faecal transfers is now being explored to treat cancers. This new treatment strategy is the result of growing evidence that the microbiota can play an important role in cancer development and progression [6][7].
Each specific region of the human body has its specific microbiome. Those areas with the richest microbiome include the skin, airways, urogenital tract, eyes and gastrointestinal tract [1]. Microbes present in the human body can also interact with the nervous system in various ways. These include via the enteric nervous system, the vagus nerve, microbial metabolites and the immune system [8][9]. Recently, the fact that the microbiome is able to interact with the nervous system and the fact that changes in the microbiota can promote the development of cancer or indeed help prevent or treat cancer, has led to the suggestion that these pathways may be mechanistically connected. Their interaction can be facilitated through the effects of the microbiome on the immune system [10]. A downregulated immune response provides favourable conditions for the development and progression of cancer [11]. A growing body of research demonstrates that changes to the composition of the gastrointestinal microbiota is an initiating factor in numerous neurocognitive conditions, profoundly influencing both CNS immunity and the integrity of the blood–brain barrier (BBB) [12].
It is now well known that the growth and progression of cancer can occur through the support of nerve tissue (neoneurogenesis) in the same way that new blood vessels (angiogenesis), or lymph vessels (lymphangiogenesis) support cancer development and progression. Additionally, in the same way, nerves are able to provide a means for cancer to metastasize and invade new tissues. Here, nerves may provide a “pathway” along which cancer cells can migrate [13]. Nerves interact with multiple tissues throughout the body, through direct interaction or via chemokines, cytokines, hormones, and neurotransmitters. Tumour cells generally have receptors for these different molecules and can also secrete a wide array of them. This provides a means for the nervous system to interact with and promote tumour development [13].
This review will discuss the role that the microbiome plays in the development and progression of various cancers, through the effect of the microbiota on the nervous system.
2. Contribution of Microbes to Hallmarks of Cancer
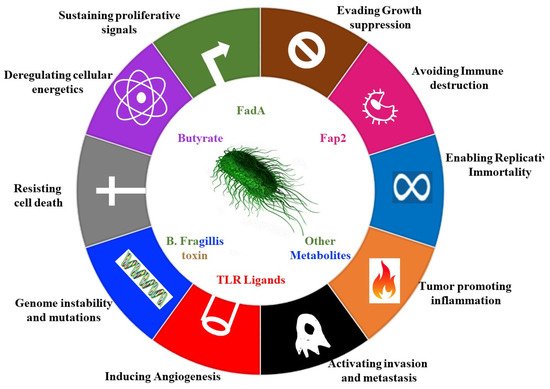
Microbes can also stimulate cancer promoting signalling pathways, such as the e-cadherin– Wnt–b-catenin signalling pathways [14][15]. Enterotoxigenic Bacteroides fragilis (ETBF) , secretes the B. fragilis toxin (BFT) (Table 1), which accelerates the endogenous cleavage of e-cadherin [16]. ß-catenin, normally bound to e-cadherins, is then liberated, and translocates to the nucleus to promote transcription of c-Myc, leading to epithelial cell proliferation ( Figure 1 ) [16].
| Metabolites | |||
|---|---|---|---|
| Anti-Cancer | |||
| Organism | Activity | Mechanism | Ref |
| Enterococcus faecalis | Produces extracellular superoxide | Cell damaging ROS | [18] |
| Helicobacter pylori or Bacteroides fragilis | Activate the host’s spermine oxidase, which, in turn, promotes gastric cancer | Generates hydrogen peroxide and reactive oxygen species | [19] |
| Lactobacillus casei | Ferrochrome | Trigger apoptosis in tumor cells via JNK pathway | [20] |
| Bacteria of the genus Propionibacteria | Butyrate, propionate | Inhibit histone deacetylase | [21] |
| Pro-oncogenic | |||
| Organism | Activity | Mechanism | Ref |
| Akkermansia muciniphila | Degrade mucin leading to changes in the levels of metabolites | Higher levels in colon cancer | [22] |
| Enerococcus faecalis | Superoxide | ROS production | [21] |
| B. acteroides fragilis |
MP toxin | B catenin pathway activation ROS production | [21] |
| Clostridium coccoides | Β-glucuronidase- | Deconjugates liver-catabolized and plant-derived estrogen’s, enabling them to bind and activate the estrogen receptors expressed by target cells |
[23] |
| Clostridium leptum | Β-glucuronidase- | Deconjugates liver-catabolized and plant-derived estrogen’s, enabling them to bind and activate the estrogen receptors expressed by target cells |
[23] |
| Escherichia coli | Colibactin and cytolethal distending toxin (CDT) | Generate DNA double-strand breaks genomic mutations | [23] |
| Fusobacterium nucleatum | FadA | Amplify tumorigenesis through E-cadherin–Wnt–β-catenin signaling | [15] |
| Helicobacter pylori | CagA | p53 degradation, B catenin MAPK, AKT pathway activation, ROS production | [21] |
| Salmonella strains | AvrA | Amplify tumorigenesis through E-cadherin–Wnt–β-catenin signaling | [24] |
| Salmonella enterico | AvrA | B catenin MAPK AKT pathway activation | [21] |
| Shigella flexneri | IpgD VirA |
p53 degradation | [21] |
| Immune related activity | |||
| Organism | Activity | Mechanism | Ref |
| Clostridium orbiscindens | TLR3 signaling | TLR3-mediated INF-β secretion by DCs in the intestine |
[25] |
| Lactobacillus acidophilus | TLR2 signaling | Anti-viral responses via TLR2-dependent IFN-β in murine bone marrow-derived DCs | [26] |
| Salmonella enterica | Mon phosphoryl lipid A (MPL) | Adjuvant in anti-cancer vaccines | [21] |
| Fusobacterium nucleatum | bacterial virulence factor Fap2, able to bind and block the NK inhibitory receptor | Inhibits host’s Natural Killer (NK) cells stimulating cancer formation by blocking immune effectors that normally inhibit tumorigenesis |
[23] |
| Porphyromonas gingivalis | Activation of TLR4 to produce increased levels of cytokines | Stimulates astrocytes contributes to the formation of neuroinflammatory lesions | [27] |
Fusobacterium nucleatum achieves a similar interaction with e-cadherin via its adhesin FadA (Table 1) [24]. Since the e-cadherin–β catenin complex regulates cellular adhesion, any interference with the complex can lead to loss of cellular adhesion, increased movement of tumour cells, invasion and metastasis [28].
Complicit microbes are microbes that promote cancer but are unable to directly cause cancer. These are often involved through their production of bioactive metabolites that modulate immune function. For example, microbes associated with lung cancer can stimulate the expression of interleukin 1β (IL-1β) and interleukin-23 (IL-23), leading to inflammation and tumour cell proliferation [28]. Jin et al. used Sftpc-Cre;Kras LSL-G12D/+ ; p53 fl/fl mutation in mice to drive the expression of Cre recombinase in lung epithelial cells, thereby inducing expression of oncogenic Kras G12D and knock out of the tumor-suppressor gene p53. Jin et al. harvested the lungs from 8 and 15 week old mice maintained under two different conditions: the first group was placed under germ-free conditions and the second group was placed in specific-pathogen free conditions. Jin et al. then stained the lungs for the proliferation marker Ki-67 and showed that the lungs from mice grown in specific-pathogen free conditions were more heavily proliferating (i.e., Ki-67-positive) compared to the lungs of the mice grown in germ-free conditions, demonstrating that the presence of commensal bacteria in the mice grown in specific pathogen free conditions exacerbated the proliferation of tumor cells. When Jin et al. examined the bronchoalveolar lavage fluid (BALF) from all mice and submitted BALF to 16sRNA qPCR, they identified the genera Staphylococcus, Streptococcus, Lactobacillus and Pasteurellaceae. Furthermore, the specific pathogen free mice had elevated levels of the cytokines Il-1β and Il-23 compared to the germ-free mice, indicating the commensal bacteria were stimulating cytokine production. Using FACS, they showed the GF mice had elevated numbers of γδT cells and the elevated γδT cells localized specifically in the lungs, where the γδT cells were responsible for producing the majority of the Il-17 pro-inflammatory cytokine. When the SP-free mice were treated with the UC7-13D T Cell receptor antibody, which acts against the γδT cells, the number of neutrophils was decreased, showing the elevated γδT cells promote neutrophil infiltration. Jin et al. argued that the commensal bacteria can exacerbate tumors via stimulating the proliferation of γδT cells, which also produce pro-inflammatory cytokines like CXC2 and IL17 and promote neutrophils to infiltrate lungs [29].
3. Neurotransmitters in Cancer and the Microbiome
The monoamine neurotransmitter, Serotonin or 5-hydroxytryptamine (5-HT) is able to act on the central nervous system (CNS), neuroendocrine system (enteric nervous system) [30][31] and the immune system [32]. It is known that serotonin interacts with the microbiome and plays a role in the development and progression of various cancers [33]. Contradictory to this, lower levels of serotonin may also promote colon cancer development, as low levels of serotonin are accompanied by increased levels of DNA damage, increased inflammation, and consequently increased levels of CRC development [34]. The production of much of the serotonin within the body is regulated by the gut microbiota. The enterochromaffin cells located in the gut supply serotonin to the mucosa, lumen, and circulating platelets and these cells are stimulated to produce serotonin through the actions of spore-forming bacteria [31]. Germ free male mice were also found to have higher levels of serotonin in their hippocampi. This is preceded by an increase in the tryptophan in the blood of the male rats, which is the precursor of serotonin [35].
In addition, serotonin stimulates proliferation in various cancers such as gliomas (where it also plays a role in migration) [36], prostate cancer [37], bladder cancer [38], small cell lung carcinoma [39], colon cancer [40], breast cancer [41], and hepatocellular carcinoma [42]. One of the processes affected by serotonin that contributes to cancer development and progression is angiogenesis. Increased serotonin leads to increased blood vessel development and an increase in the size of blood vessels [43][44]. Studies have also focused on the use of altered serotonin or serotonin receptor [45] expression patterns as a diagnostic or prognostic biomarker in various cancers including urological cancers [45], and colon cancer [46]. The receptors most commonly associated with the development and progression of cancers are the 5-HT 1 and 5-HT 2 receptors [47][48][49]. Activation of these receptors alters the progression of the cell cycle, stimulates cell growth and results in improved cell viability this is due to the activation of genes such as MEK-ERK1/2 and the JAK2-STAT3 signalling pathways [43]. Increased expression of these receptors has been identified in ovarian [50], and prostate cancer [51]. In some cases, antagonists of serotonin receptors, inhibitors of selective serotonin transporter and of serotonin synthesis have been successfully used to prevent cancer cell growth in prostate cancer [52].
The migration of cancer cells was found to be stimulated by neurobiological signals, namely through the signalling of norepinephrine [53]. The correct levels of the neurotransmitter may depend on the correct populations of bacteria within the gut as germ free mice have significantly lower levels of norepinephrine [54]. In addition to dopamine stimulating dopaminergic neurons, they activate the innate and adaptive immune cells [55]. The implications of the activation of the immune systems in the development of cancer has already been discussed. Dopamine is also synthesized and secreted by various bacteria [56].
Bacteria from the genera Lactobacillus and Bifidobacterium are able to produce the neurotransmitter gamma-aminobutyric acid (GABA) [57]. GABA binds to the serpentine receptor GABA(B) and the signal is internalised through a decrease in the level of the cyclic AMP [57]. GABA was found to reduce the migration of colon cancer cells in culture through modulating the activity of norepinephrine [53].
4. Neurogenesis and miRNA Regulation by Microbiota
The creation of new nervous tissue (neurogenesis) is an important process for the progression of most cancers. Tumour cells produce factors that lead to the formation of new nerve tissue [58]. These newly formed nerves release neurotransmitters that stimulate tumour growth and migration [59]. Cancers can invade new tissue and migrate along nerves or nerve tissue. Like angiogenesis and lymphogenesis these new nerves also support the new tumour leading to the growth of cancers around these new nerves in a process known as perineural invasion (PNI) [60]. The microbiome is also able to initiate signalling cascades which stimulate neurogenesis by activating TLR2. The process of neurogenesis can be inhibited, delayed, or even counteracted by feeding animals a mixture of specific bacteria that changes the populations of their gut microbiota [61][62].
The regulation of gene expression through the actions of miRNA is known to play a role in neuronal proliferation, neurogenesis, and Brain Derived Neurotrophic Factor (BDNF) signalling. These processes as well as the expression of certain miRNAs is altered in germ free mice [63]. Studies involving the next generation sequencing of miRNA from normal, germ free and antibiotic treated mice indicate that miRNA expression in the amygdala and prefrontal cortex is regulated by the microbiota and changes in the microbiota populations result in changes in the expression of miRNA. The miRNA expression pattern of germ-free mice was altered once again following bacterial colonisation of the germ-free mice [64], One of the miRNAs whose expression is targeted by gut microbiota is miR-206-3p. This miRNA is known to regulate the expression of the neurotrophic BDNF [64][65]. BDNF is known to stimulate growth of neurons and is important for the cancer related neurogenesis that is also involved in the invasion, metastasis and support of cancer development and growth (Reviewed in [66]).
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220.
- Hoption Cann, S.A.; van Netten, J.P.; van Netten, C. Dr William Coley and tumour regression: A place in history or in the future. Postgrad. Med. J. 2003, 79, 672–680.
- Bickels, J.; Kollender, Y.; Merinsky, O.; Meller, I. Coley’s toxin: Historical perspective. Isr. Med. Assoc. J. 2002, 4, 471–472.
- Starnes, C.O. Coley’s toxins in perspective. Nature 1992, 357, 11–12.
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204.
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44.
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335.
- Mangani, D.; Weller, M.; Roth, P. The network of immunosuppressive pathways in glioblastoma. Biochem. Pharmacol. 2017, 130, 1–9.
- Subramaniam, C.B.; Bowen, J.M.; Gladman, M.A.; Lustberg, M.B.; Mayo, S.J.; Wardill, H.R. The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci. Biobehav. Rev. 2020, 116, 470–479.
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157.
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123.
- Silva-García, O.; Valdez-Alarcón, J.J.; Baizabal-Aguirre, V.M. Wnt/β-Catenin Signaling as a Molecular Target by Pathogenic Bacteria. Front. Immunol. 2019, 10, 2135.
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Huycke, M.M.; Abrams, V.; Moore, D.R. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 2002, 23, 529–536.
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474.
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365.
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38.
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597.
- Lara-Tejero, M.; Galán, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 2000, 290, 354–357.
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206.
- He, Z.; Gharaibeh, R.Z.; Newsome, R.C.; Pope, J.L.; Dougherty, M.W.; Tomkovich, S.; Pons, B.; Mirey, G.; Vignard, J.; Hendrixson, D.R.; et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2019, 68, 289–300.
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frøkiaer, H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 2010, 131, 268–281.
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970.
- Beavon, I.R. The E-cadherin-catenin complex in tumour metastasis: Structure, function and regulation. Eur. J. Cancer 2000, 36, 1607–1620.
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013.e1016.
- Chin, A.; Svejda, B.; Gustafsson, B.I.; Granlund, A.B.; Sandvik, A.K.; Timberlake, A.; Sumpio, B.; Pfragner, R.; Modlin, I.M.; Kidd, M. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G397–G405.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276.
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728.
- Singhal, M.; Turturice, B.A.; Manzella, C.R.; Ranjan, R.; Metwally, A.A.; Theorell, J.; Huang, Y.; Alrefai, W.A.; Dudeja, P.K.; Finn, P.W.; et al. Serotonin Transporter Deficiency is Associated with Dysbiosis and Changes in Metabolic Function of the Mouse Intestinal Microbiome. Sci. Rep. 2019, 9, 2138.
- Sakita, J.Y.; Bader, M.; Santos, E.S.; Garcia, S.B.; Minto, S.B.; Alenina, N.; Brunaldi, M.O.; Carvalho, M.C.; Vidotto, T.; Gasparotto, B.; et al. Serotonin synthesis protects the mouse colonic crypt from DNA damage and colorectal tumorigenesis. J. Pathol. 2019, 249, 102–113.
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673.
- Merzak, A.; Koochekpour, S.; Fillion, M.P.; Fillion, G.; Pilkington, G.J. Expression of serotonin receptors in human fetal astrocytes and glioma cell lines: A possible role in glioma cell proliferation and migration. Brain Res. Mol. Brain Res. 1996, 41, 1–7.
- Siddiqui, E.J.; Shabbir, M.; Mikhailidis, D.P.; Thompson, C.S.; Mumtaz, F.H. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J. Urol. 2006, 176, 1648–1653.
- Siddiqui, E.J.; Shabbir, M.A.; Mikhailidis, D.P.; Mumtaz, F.H.; Thompson, C.S. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 2006, 97, 634–639.
- Cattaneo, M.G.; Palazzi, E.; Bondiolotti, G.; Vicentini, L.M. 5-HT1D receptor type is involved in stimulation of cell proliferation by serotonin in human small cell lung carcinoma. Eur. J. Pharmacol. 1994, 268, 425–430.
- Tutton, P.J.; Steel, G.G. Influence of biogenic amines on the growth of xenografted human colorectal carcinomas. Br. J. Cancer 1979, 40, 743–749.
- Pai, V.P.; Marshall, A.M.; Hernandez, L.L.; Buckley, A.R.; Horseman, N.D. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009, 11, R81.
- Soll, C.; Jang, J.H.; Riener, M.O.; Moritz, W.; Wild, P.J.; Graf, R.; Clavien, P.A. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 2010, 51, 1244–1254.
- Oufkir, T.; Arseneault, M.; Sanderson, J.T.; Vaillancourt, C. The 5-HT 2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta 2010, 31, 439–447.
- Zamani, A.; Qu, Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 2012, 586, 2360–2365.
- Jungwirth, N.; Haeberle, L.; Schrott, K.M.; Wullich, B.; Krause, F.S. Serotonin used as prognostic marker of urological tumors. World J. Urol. 2008, 26, 499–504.
- Ataee, R.; Ajdary, S.; Zarrindast, M.; Rezayat, M.; Shokrgozar, M.A.; Ataee, A. Y25130 hydrochloride, a selective 5HT3 receptor antagonist has potent antimitogenic and apoptotic effect on HT29 colorectal cancer cell line. Eur. J. Cancer Prev. 2010, 19, 138–143.
- Ataee, R.; Ajdary, S.; Rezayat, M.; Shokrgozar, M.A.; Shahriari, S.; Zarrindast, M.R. Study of 5HT3 and HT4 receptor expression in HT29 cell line and human colon adenocarcinoma tissues. Arch. Iran. Med. 2010, 13, 120–125.
- Nishikawa, T.; Tsuno, N.H.; Shuno, Y.; Sasaki, K.; Hongo, K.; Okaji, Y.; Sunami, E.; Kitayama, J.; Takahashi, K.; Nagawa, H. Antiangiogenic effect of a selective 5-HT4 receptor agonist. J. Surg. Res. 2010, 159, 696–704.
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.J.; Vaillancourt, C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059.
- Henriksen, R.; Dizeyi, N.; Abrahamsson, P.A. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012, 32, 1361–1366.
- Dizeyi, N.; Bjartell, A.; Hedlund, P.; Taskén, K.A.; Gadaleanu, V.; Abrahamsson, P.A. Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur. Urol. 2005, 47, 895–900.
- Abdul, M.; Anezinis, P.E.; Logothetis, C.J.; Hoosein, N.M. Growth inhibition of human prostatic carcinoma cell lines by serotonin antagonists. Anticancer Res. 1994, 14, 1215–1220.
- Joseph, J.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002, 62, 6467–6469.
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119.
- Ben-Shaanan, T.L.; Azulay-Debby, H.; Dubovik, T.; Starosvetsky, E.; Korin, B.; Schiller, M.; Green, N.L.; Admon, Y.; Hakim, F.; Shen-Orr, S.S.; et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 2016, 22, 940–944.
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726.
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417.
- Entschladen, F.; Palm, D.; Lang, K.; Drell, T.L.t.; Zaenker, K.S. Neoneurogenesis: Tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 2006, 67, 33–35.
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408.
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520.
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, e7–e9.
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155.
- Hoban, A.E.; Stilling, R.; Moloney, G.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 2017, 5, 102.
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.H.; Huh, J.Y.; Yoon, H.; Park, D.K.; Lim, J.Y.; Kim, J.M.; Jeon, D.; et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 2012, 72, 269–277.
- Dlamini, Z.; Mathabe, K.; Padayachy, L.; Marima, R.; Evangelou, G.; Syrigos, K.N.; Bianchi, A.; Lolas, G.; Hull, R. Many Voices in a Choir: Tumor-Induced Neurogenesis and Neuronal Driven Alternative Splicing Sound Like Suspects in Tumor Growth and Dissemination. Cancers 2021, 13, 2138.




