
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel López | + 1837 word(s) | 1837 | 2021-09-07 08:04:26 | | | |
| 2 | Dean Liu | Meta information modification | 1837 | 2021-10-26 05:12:07 | | |
Video Upload Options
Chikungunya virus (CHIKV) is an Alphavirus of the Togaviridae family that is transmitted by mosquitoes of the genus Aedes. The virus causes acute febrile illness in infected people, which frequently leads to chronic debilitating polyarthritis and polyarthralgia. Most of the symptoms resolve after 10 days, but polyarthralgia can persist for months or years, and severe symptoms, such as encephalitis, hemorrhagic disease and mortality, have also been described.
1. Vaccinia Virus and the Success against Smallpox
Smallpox is a highly contagious disease that has plagued humankind as one of the most lethal pandemics, with a persistent and universal impact on the human population since its probable emergence in the first irrigated agricultural settlements. Nearly one billion deaths have been attributed to variola virus, with approximately 300–500 million deaths in the 20th century [1]. This pathogen severely altered the course of history at different times, contributing to the decline of some human civilizations, with a 30% mortality rate [2]. Early efforts to protect people against severe forms of smallpox by inoculation with smallpox scabs and/or pus (variolation) have been historically documented from medieval times in China and India. From these cultures, the variolation technique slowly extended to southwestern Asia, Japan, the Middle East and the Ottoman Empire, and arrived later to Africa, Europe and European colonies around the world [3]. Although immunization by variolation was a reasonably effective and preventive medical procedure against smallpox, some subjects inoculated with these secretions from infectious variola virus became seriously ill or died. In addition, these individuals could initiate smallpox outbreaks in susceptible populations.
After the low vulnerability of milkmaids to smallpox was observed in rural areas of European countries, Edward Jenner and other English, Dutch and German physicians inoculated different study subjects with fluid extracted from pustules on the hands and arms of cowpox- or horsepox-infected milkmaids [3][4]. This prophylactic measure was so successful that inoculation with pustule fluid quickly spread in most European countries, their overseas colonies and the newly independent United States of America (USA), starting the era of prophylactic vaccines. A remarkable historic episode in this field was the first mass vaccination campaign against smallpox, launched by Spain in 1803. In this expedition, 22 orphan boys from Galicia were utilized during the sea voyage as successive carriers of the Jenner vaccine to America. In addition, the expedition also carried scientific instruments and Spanish translations of one recent book on vaccination to be distributed to the local vaccine commissions, organized by the expedition members as they visited the different territories [5][6]. Other independent initiatives to vaccinate the American population were also successful. For example, Dr. Benjamin Waterhouse introduced the vaccine in Boston, and the vaccine was brought to California in 1817 by Russian merchants who obtained it in Peru [6].
The isolation of pustule fluids from very heterogeneous origins in different European countries and their random exchange throughout the 19th century generated complex mixtures of a vaccine [7]. The main vaccine utilized in the massive worldwide vaccination program organized by the WHO that eradicated smallpox was vaccinia virus (VACV) [8].
2. Vaccinia Virus and the Success against Smallpox
Smallpox is a highly contagious disease that has plagued humankind as one of the most lethal pandemics, with a persistent and universal impact on the human population since its probable emergence in the first irrigated agricultural settlements. Nearly one billion deaths have been attributed to variola virus, with approximately 300–500 million deaths in the 20th century [1]. This pathogen severely altered the course of history at different times, contributing to the decline of some human civilizations, with a 30% mortality rate [2]. Early efforts to protect people against severe forms of smallpox by inoculation with smallpox scabs and/or pus (variolation) have been historically documented from medieval times in China and India. From these cultures, the variolation technique slowly extended to southwestern Asia, Japan, the Middle East and the Ottoman Empire, and arrived later to Africa, Europe and European colonies around the world [3]. Although immunization by variolation was a reasonably effective and preventive medical procedure against smallpox, some subjects inoculated with these secretions from infectious variola virus became seriously ill or died. In addition, these individuals could initiate smallpox outbreaks in susceptible populations.
After the low vulnerability of milkmaids to smallpox was observed in rural areas of European countries, Edward Jenner and other English, Dutch and German physicians inoculated different study subjects with fluid extracted from pustules on the hands and arms of cowpox- or horsepox-infected milkmaids [3][4]. This prophylactic measure was so successful that inoculation with pustule fluid quickly spread in most European countries, their overseas colonies and the newly independent United States of America (USA), starting the era of prophylactic vaccines. A remarkable historic episode in this field was the first mass vaccination campaign against smallpox, launched by Spain in 1803. In this expedition, 22 orphan boys from Galicia were utilized during the sea voyage as successive carriers of the Jenner vaccine to America. In addition, the expedition also carried scientific instruments and Spanish translations of one recent book on vaccination to be distributed to the local vaccine commissions, organized by the expedition members as they visited the different territories [5][6]. Other independent initiatives to vaccinate the American population were also successful. For example, Dr. Benjamin Waterhouse introduced the vaccine in Boston, and the vaccine was brought to California in 1817 by Russian merchants who obtained it in Peru [6].
The isolation of pustule fluids from very heterogeneous origins in different European countries and their random exchange throughout the 19th century generated complex mixtures of a vaccine [7]. The main vaccine utilized in the massive worldwide vaccination program organized by the WHO that eradicated smallpox was vaccinia virus (VACV) [8].
3. Third-Generation Smallpox Vaccines: MVA
At this point, the development of attenuated smallpox vaccines for people with contraindications to traditional smallpox vaccines was required. Among other approaches, the wild-type chorioallantois VACV Ankara (CVA) strain was serially passaged on chick embryo fibroblasts over 516 times during late 1950s and early 1960s, and renamed the modified vaccinia virus Ankara (MVA) strain [9]. In addition, during the 1970s, the process continued, and MVA reached more than 570 passages. During this long in vitro passage, six major deletions and multiple other alterations were identified in this new modified vaccinia virus Ankara (MVA) strain when compared to the genome of its parental CVA [10]. For example, 124 MVA open reading frames (ORFs) encode proteins that contain one or various amino acid exchanges or insertions/deletions compared with the gene products encoded by orthologous CVA ORFs [11]. These changes severely impede the replication of MVA in mammalian cells, but not in chick embryo fibroblasts [12]. In mammalian cells, an MVA replication blockade occurs after immature virions are formed; thus, virus-infected cells express and accumulate high levels of MVA-encoded proteins, allowing MVA to have a good immunogenicity profile and diminished virulence in mammalian hosts [13][14]. In some specialized cells, such as macrophages and dendritic cells that do not allow late gene expression, antigen generation is low [12][15]. Overall, MVA is generally referred to as a nonreplicating viral vector in human cells. Therefore, MVA is an alternative smallpox vaccine [16][17] that was used in Germany in the 1970s, close to the end of the WHO smallpox campaign [18], and was approved by the US Food and Drug Administration (FDA) on 24 September 2019, to prevent both smallpox and monkeypox. It was also approved for human use under specifications against smallpox in Europe by the European Medicines Agency (EMA) and in Canada. In addition, MVA can be lyophilized, which allows for easier and less expensive transportation, storage and distribution without cold chains, which are essential requirements in developing countries, where transport and health infrastructures are deficient. Moreover, recombinant MVA has also been used in several preclinical and human clinical trials as a vaccine candidate against numerous human infectious diseases, such as HIV/AIDS, malaria, tuberculosis, hepatitis C, emerging viruses (such as chikungunya, Zika and Ebola) and even against several tumors [19][20]. All these preclinical and clinical trials convert MVA into a reliable vaccine platform that can be used against any viral pathogen.
4. History, Pathology and Structure of Chikungunya Virus
Chikungunya virus (CHIKV) is an Alphavirus of the Togaviridae family that is transmitted by mosquitoes of the genus Aedes [21]. The virus causes acute febrile illness in infected people, which frequently leads to chronic debilitating polyarthritis and polyarthralgia. Most of the symptoms resolve after 10 days, but polyarthralgia can persist for months or years [22], and severe symptoms, such as encephalitis, hemorrhagic disease and mortality, have also been described [23].
This arboviral pathogen was discovered in Tanzania under British colonial administration in 1952 [24]. In the following years, several outbreaks were identified in other British colonies in Africa, probably facilitated by the political and commercial relations between the different British territories. Later, this pathogen caused frequent epidemics in Africa and Asia from the 1960s to the 1980s [25]. The relative inactivity of this virus for the following 15 years ended in 2005 with an explosive epidemic in the French overseas department of La Réunion and other Indian Ocean islands, with more than 700,000 cases and 250 deaths [26]. In 2006, several million people were affected by this pathogen in a new massive outbreak in India [27]. Since then, CHIKV has expanded rapidly to practically all tropical and subtropical regions of the world [28], with increasing severity compared with that previously reported [29]. In recent years, Italy and other European countries have also reported CHIKV outbreaks [30]. Therefore, morbidity due to this virus is a serious threat to global health, making CHIKV a high-priority emerging pathogen [31].
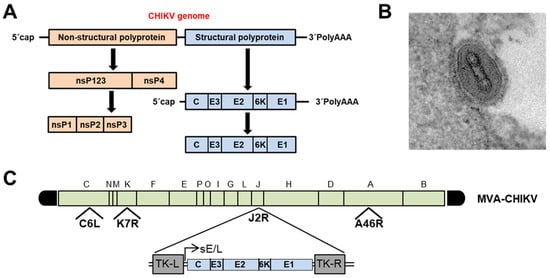
The CHIKV capsid encloses an approximately 12-kb single-stranded, positive-sense RNA genome that codes for two large polyproteins [32] ( Figure 1 A). The first is the nonstructural P1234 precursor, which is autocatalytically processed by the C-terminal domain of nonstructural protein 2 (nsP2), releasing the four multifunctional nsP proteins. Moreover, the maturation of the structural polyprotein involves three different proteases. First, the capsid (C) is autocatalytically released, and later, two host proteases (the endoplasmic reticulum (ER) signal peptidase and furin proteases) generate the 6K transmembrane and the three E1, E2, and E3 envelope proteins [32].
References
- Henderson, D.A. The eradication of smallpox—An overview of the past, present, and future. Vaccine 2011, 29 (Suppl. 4), D7–D9.
- Barquet, N.; Domingo, P. Smallpox: The triumph over the most terrible of the ministers of death. Ann. Intern. Med. 1997, 127, 635–642.
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I. Smallpox and Its Eradication; WHO: Geneva, Switzerland, 2004.
- Esparza, J.; Schrick, L.; Damaso, C.R.; Nitsche, A. Equination (inoculation of horsepox): An early alternative to vaccination (inoculation of cowpox) and the potential role of horsepox virus in the origin of the smallpox vaccine. Vaccine 2017, 35, 7222–7230.
- Mark, C.; Rigau-Perez, J.G. The world’s first immunization campaign: The Spanish Smallpox Vaccine Expedition, 1803–1813. Bull. Hist. Med. 2009, 83, 63–94.
- Esparza, J. Three different paths to introduce the smallpox vaccine in early 19th century United States. Vaccine 2020, 38, 2741–2745.
- Qin, L.; Upton, C.; Hazes, B.; Evans, D.H. Genomic analysis of the vaccinia virus strain variants found in Dryvax vaccine. J. Virol. 2011, 85, 13049–13060.
- Sanchez-Sampedro, L.; Perdiguero, B.; Mejias-Perez, E.; Garcia-Arriaza, J.; Di, P.M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803.
- Mayr, A.; Munz, E. Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures. Zentralbl. Bakteriol. Orig. 1964, 195, 24–35.
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F.G. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396.
- Meisinger-Henschel, C.; Schmidt, M.; Lukassen, S.; Linke, B.; Krause, L.; Konietzny, S.; Goesmann, A.; Howley, P.; Chaplin, P.; Suter, M.; et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J. Gen. Virol. 2007, 88 Pt 5, 3249–3259.
- Blanchard, T.J.; Alcami, A.; Andrea, P.; Smith, G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: Implications for use as a human vaccine. J. Gen. Virol. 1998, 79, 1159–1167.
- Sutter GMoss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851.
- Sutter, G.; Wyatt, L.S.; Foley, P.L.; Bennink, J.R.; Moss, B. A recombinant vector derived from the host range- restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine 1994, 12, 1032–1040.
- Gomez, C.E.; Najera, J.L.; Krupa, M.; Esteban, M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 2008, 8, 97–120.
- Kennedy, J.S.; Greenberg, R.N. IMVAMUNE: Modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert. Rev. Vaccines 2009, 8, 13–24.
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908.
- Stickl, H.; Hochstein-Mintzel, V.; Mayr, A.; Huber, H.C.; Schafer, H.; Holzner, A. MVA vaccination against smallpox: Clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl). Dtsch. Med. Wochenschr. 1974, 99, 2386–2392.
- Gomez, C.E.; Perdiguero, B.; Garcia-Arriaza, J.; Esteban, M. Poxvirus vectors as HIV/AIDS vaccines in humans. Hum. Vaccin Immunother. 2012, 8, 1192–1207.
- Tomori, O.; Kolawole, M.O. Ebola virus disease: Current vaccine solutions. Curr. Opin. Immunol. 2021, 71, 27–33.
- Her, Z.; Kam, Y.W.; Lin, R.T.; Ng, L.F. Chikungunya: A bending reality. Microbes. Infect. 2009, 11, 1165–1176.
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le, G.R.; Gras, G.; Roques, P. Chikungunya disease: Infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446.
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500.
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–1953. I. Clinical features. Trans. R Soc. Trop. Med. Hyg. 1955, 49, 28–32.
- Halstead, S.B.; Scanlon, J.E.; Umpaivit, P.; Udomsakdi, S. Dengue and chikungunya virus infection in man in Thailand, 1962-1964, IV. Epidemiologic studies in the Bangkok metropolitan area. Am. J. Trop. Med. Hyg. 1969, 18, 997–1021.
- Powers, A.M.; Logue, C.H. Changing patterns of chikungunya virus: Re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007, 88, 2363–2377.
- Saxena, S.K.; Singh, M.; Mishra, N.; Lakshmi, V. Resurgence of chikungunya virus in India: An emerging threat. Eurosurveillance 2006, 11, E060810.
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. 2016, 17, 671–679.
- Pastorino, B.; Muyembe-Tamfum, J.J.; Bessaud, M.; Tock, F.; Tolou, H.; Durand, J.P.; Peyrefitte, C.N. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: Identification of a new central African strain. J. Med. Virol. 2004, 74, 277–282.
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47.
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239.
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197.
- Gallego-Gomez, J.C.; Risco, C.; Rodriguez, D.; Cabezas, P.; Guerra, S.; Carrascosa, J.L.; Esteban, M. Differences in virus-induced cell morphology and in virus maturation between MVA and other strains (WR, Ankara, and NYCBH) of vaccinia virus in infected human cells. J. Virol. 2003, 77, 10606–10622.
- Goebel, S.J.; Johnson, G.P.; Perkus, M.E.; Davis, S.W.; Winslow, J.P.; Paoletti, E. The complete DNA sequence of vaccinia virus. Virology 1990, 179, 247–263.
- Chakrabarti, S.; Sisler, J.R.; Moss, B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques 1997, 23, 1094–1097.
- Garcia-Arriaza, J.; Cepeda, V.; Hallengard, D.; Sorzano, C.O.; Kummerer, B.M.; Liljestrom, P.; Esteban, M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014, 88, 3527–3547.




