As part of a project devoted to the phytochemical study of Ecuadorian biodiversity, new essential oils are systematically distilled and analysed. In the present work, Jungia rugosa Less (Asteraceae) has been selected and some wild specimens collected to investigate the volatile fraction. The essential oil, obtained from fresh leaves, was analysed for the first time in the present study. The chemical composition was determined by gas chromatography, coupled to mass spectrometry (GC-MS) for qualitative analysis, and to flame ionization detector (GC-FID) for quantitation. The calculation of relative response factors (RRF), based on combustion enthalpy, was carried out for each quantified component. Fifty-six compounds were identified and quantified in a 5% phenyl-polydimethylsiloxane non-polar column and 53 compounds in a polyethylene glycol polar column, including four undetermined compounds. The main feature of this essential oil was the exclusive sesquiterpenes content, both hydrocarbons (74.7% and 80.4%) and oxygenated (8.3% and 9.6%). Major constituents were: γ-curcumene (47.1% and 49.7%) and β-sesquiphellandrene (17.0% and 17.9%), together with two abundant undetermined oxygenated sesquiterpenes, whose abundance was 6.7–7.2% and 4.7–3.3%, respectively.
1. Introduction
Ecuador, due to multiple combinations of factors, has been configured as a megadiverse country, with a high rate of plant endemism per surface area, which makes it one of the richest countries in biodiversity and endemism of the world
[1][2]. Some figures presented in the Fifth and Sixth National Report for the Convention on Biological Diversity regarding the emergence of new plant species illustrate this peculiarity: between 1999 and 2012, 2443 new species were reported for the country, of which 1663 were also new to the science. In 2013, 18,198 species of vascular plants were registered, which meant 1140 more species than those reported in 2010 and representing about 7.6% of the vascular plants registered worldwide. It is estimated that the total number of vascular plants could reach 25,000
[3][4].
Along with the above, indigenous cultures possess a strong tradition about plants as a means of treating diseases, which has allowed ancestral knowledge to be transferred through generations from ancient times to the present, promoting the abundant use of medicinal plants. For all these reasons, Ecuador is an invaluable source of natural products and unprecedented knowledge about plant applications. In contrast, the number of high-impact scientific studies in this area is relatively low, given the potential that the country’s biodiversity offers
[5]. In this respect, to the best of the authors’ knowledge, the essential oil (EO), distilled from the leaves of
Jungia rugosa Less, has never been described.
Within the Asteraceae, the
Jungia genus corresponds to flowering plants that mostly develop at high altitudes and cold climates, being characteristic of the Andean regions of Ecuador, Peru, and Argentina. Despite many articles describing the phytochemistry of genus
Jungia, only three deal with EOs. In fact, only the volatile fractions of
Jungia paniculata and
Jungia polita have been described so far, the first one being very popular in the Andes and known with the traditional name “matico”
[6][7][8]. Concerning
J. rugosa, two phytochemical studies have been published. However, they are devoted to non-volatile fractions and their biological activities
[9][10].
Jungia rugosa Less (Asteraceae) is a native Andean species, growing at altitudes between 1500 and 4000 m above sea level
[11]. It is characterised by great resistance to frost and low temperatures, which is why it prevails in cold and humid climates. This plant grows up to 5 m in height, presenting a thin, woody, smooth, hard, and green stem. Its intense green leaves with a pale green underside, measure between 5 and 12 centimetres and are covered with villi; they are also petiolate, presenting an anti-parallel rib. Its main root divides, giving rise to an abundant root system. Its flower is whitish in colour, presented in a green capsule, which generates small black seeds. In some localities located in the Andean region of Ecuador, it is better known as “carne humana”. Based on the indigenous heritage of the central Ecuadorian region (Cotopaxi), this species is used as an anti-inflammatory remedy, for instance, in treating bruises, and for other unspecified healing purposes
[12]. The anti-inflammatory activity is probably the most important medicinal property of this plant, since it has also been confirmed by two scientific studies, together with the closely related antioxidant capacity
[13][14]. Some sources also report that leaf decoctions are applied to treat wounds and skin ulcerations, gastric problems, and kidney disorders, among others
[15][16]. In addition to medicinal applications, this species is also used to prepare ropes in the Chimborazo region of Ecuador
[12]. Furthermore,
J. rugosa is also known with three botanical synonyms:
Jungia bullata Turcz.,
Jungia jelskii Hieron., and
Jungia malvifolia Muschl
[17]. None of these synonyms corresponds to any chemical literature.
So far, many plant species from Ecuador have been described for producing new EOs, often characterised by important biological activities such as analgesic, antioxidant, antibacterial, anticancer, and sedative, among others
[5][18][19][20]. In particular, EOs rich in sesquiterpenes have been presented as promising anti-proliferative agents, whose constituents are able to easily reach certain organs, such as heart, liver, and kidneys
[20]. Among all the biological properties of EOs and their constituents, we are particularly interested in the inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), due to the serious implications that neurodegenerative diseases are ever-more producing in western countries
[18][21][22][23].
In accordance with the above, the objectives of this research were to investigate the chemical and enantiomeric composition of
J. rugosa EO and to evaluate the presence of cholinergic molecules in this volatile fraction. All this information will provide a contribution to the phytochemical and phytopharmacological knowledge of the Ecuadorian flora.
2. Discussion
2.1. The Chemical Composition
About the chemical composition of the EO, the hydrocarbon sesquiterpene fraction was predominant, corresponding to 74.7% and 80.4% with a non-polar and a polar column respectively. Furthermore, an oxygenated sesquiterpene fraction was present between 9.6% and 8.3% of the whole amount. No monoterpenes were detected in the EO. Major components of this volatile fraction were γ-curcumene and β-sesquiphellandrene, together with two undetermined oxygenated sesquiterpenes (molecular weight 220 amu and 262 amu, respectively). If we compare these results with the only two partial analyses, known so far for EOs of genus
Jungia (
J. paniculata and
J. polita), the prevalence of sesquiterpenes is confirmed
[7][8]. However, unlike our case, (
E)-β-caryophyllene and caryophyllene oxide are there the main components. Regarding γ-curcumene, it derives its name from
Curcuma longa L. (turmeric), but we must look at
Helichrysum italicum (Roth) G. Don (Asteraceae) to find an important and widely studied botanical species where γ-curcumene is often a major constituent. Other
Helichrysum species are also familiar with similar sesquiterpene compositions
[24]. On the one hand, despite γ-curcumene being quite common and known for a long time, no exhaustive studies on its pharmacology can be found. On the other hand, the EOs where it is an important component are widely described, with all the typical biological activities known for volatile fractions. In regards to β-sesquiphellandrene, it is also a typical hydrocarbon sesquiterpene of
Curcuma longa. The most important study on its pharmacological properties is probably a recent publication, where β-sesquiphellandrene has been described as a potent anticancer agent. Its activity is comparable with the one of curcumin. According to that investigation, β-sesquiphellandrene would exert an antiproliferative activity, by inhibiting the formation of cancer cell colonies and inducing apoptosis. The neoplastic formations, that appeared to be more sensitive to this metabolite, were leukaemia, multiple myeloma, and colorectal cancer. Furthermore, cancer cells expressing p-53 protein resulted in being more sensitive to β-sesquiphellandrene than those lacking it
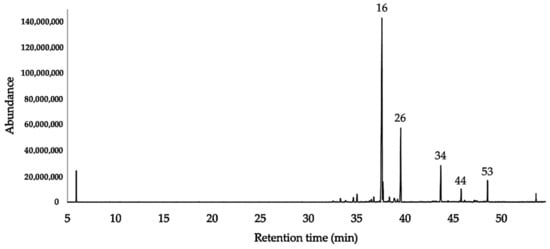
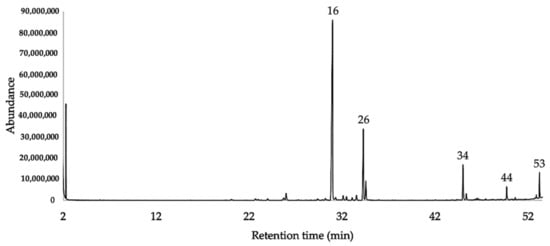
[25]. Finally, we must mention the presence of two important undetermined compounds, contributing to the mass of the EO with the non-negligible amounts of 6.7–7.2% and 4.7–3.3%, respectively (see peaks 34 and 53 in
Figure 1 and
Figure 2). These constituents showed a molecular ion of 220 and 262 amu—the first one being characteristic of sesquiterpenoids with molecular formula C
15H
24O, whereas the second one is consistent with the rare sesquiterpene derivatives of formula C
18H
30O (e.g., sesquiterpenes acetones)
[26].
Figure 1. GC-MS chromatogram of J. rugosa EO on DB-5ms column.
Figure 2. GC-MS chromatogram of J. rugosa EO on HP-INNOWax column.
2.2. The Enantiomeric Evaluation
For what concerns the enantiomeric evaluation, the EO was submitted to enantioselective GC, in a classical β-cyclodextrin-based capillary column. However, the only chiral terpene that could be identified was (1S,2R,6R,7R,8R)-(+)-α-copaene, present as a pure enantiomer. No other sesquiterpene could be detected, both as a pure enantiomer or enantiomeric pair. This result is not surprising. In fact, most of the enantiomerically pure available standards are indeed monoterpenes, whose use is necessary to determine the elution order of the enantiomers from an enantioselective column. Since the present EO is exclusively constituted by sesquiterpenes, the corresponding enantioselective GC information is, for most of them, unavailable. Furthermore, the similarity among the spectra for this class of metabolites excluded the possibility to certainly identify enantiomeric pairs within the peaks. The only exception, although a minor component, was (1S,2R,6R,7R,8R)-(+)-α-copaene, since the enantiomerically pure standards of this compound are available.
2.3. The Cholinergic Activity
Finally, the inhibition activity of this EO against AChE can be discussed. Observing our results, shown in
Table 1, the inhibition capacity of
J. rugosa EO was compared to the ones of galanthamine and
L. nobilis EO. However, whereas the biological activity of galanthamine is clearly extremely high, mainly because it is a pure compound, the biological activity of
L. nobilis EO is decidedly lower. Nevertheless,
L. nobilis EO is considered as an active mixture in this kind of assay, and it can be subsequently used as a better positive control while working with EOs
[27]. In our case, the inhibition power of
J. rugosa EO is about 68% compared to that of
L. nobilis EO, clearly resulting in less activity but not inactive. This fact could be explained by the presence of at least one active minor sesquiterpene in the mixture. If that is the case, the EO may be considered useless as it is, but suitable to be studied, through a bio-guided fractionation, in search of new sesquiterpene inhibitors. The interest in this aspect resides in that, to the best of the authors’ knowledge, the most active EOs are characterised by an important monoterpene fraction (except for the case where the EO is dominated by (
E)-β-caryophyllene)
[18][22]. However, due to their toxicity, hydrocarbon monoterpenes can hardly be used as pharmaceutical active principles, which cannot be assumed for sesquiterpenes. Therefore, the discovery of new sesquiterpene inhibitors of AChE is a matter of some pharmaceutical interest. Consequently, this volatile fraction is suitable for further investigation, according to two main lines: a) the purification and structure elucidation of the major undetermined compounds, by mean of preparative chromatography and NMR spectroscopy; b) a bio-guided preparative fractionation, intended to investigate the presence of new sesquiterpene AChE inhibitors among the minor components. Due to the low distillation yield of this EO, a non-classical approach should be applied. On the one hand, a tentative method for purification and structure elucidation could be the use of preparative thin-layer chromatography (TLC) and micro-probe NMR spectroscopy. In this way, about 1 mg of a pure compound would be enough to be submitted to a complete series of NMR experiments. On the other hand, the bio-guided investigation could be faced through a bioautographic method. Based on a TLC analysis, a bioautographic assay can be carried out with few micrograms of EO. Since the active compounds possibly are known sesquiterpenes, the combined use of bioautography, preparative TLC and GC-MS should afford the desired information.
Table 1. Percent inhibition of AChE by J. rugosa EO compared to L. nobilis EO and galantamine as positive controls.
| Sample |
Enzymatic Inhibition (%) |
σ |
| Galanthamine 1.0 µg/mL |
49.2 |
5.2 |
| Laurus nobilis EO 38 µg/mL |
38.8 |
4.2 |
| Jungia rugosa EO 38 µg/mL |
25.9 |
13.9 |
In regards to the traditional use of
J. rugosa, some previous studies have described the antioxidant and anti-inflammatory activities of the non-volatile fraction, mainly attributed to flavonoids
[13][14]. Since these properties are fully consistent with the ethnobotanical use, the EO could probably be exempted to be considered the active fraction.