
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chloë De Roo | + 1395 word(s) | 1395 | 2021-10-18 08:06:06 | | | |
| 2 | Jessie Wu | Meta information modification | 1395 | 2021-10-20 03:29:35 | | |
Video Upload Options
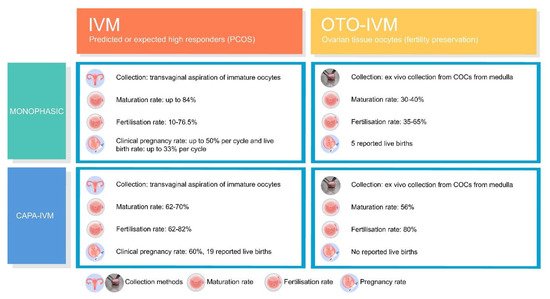
In vitro maturation (IVM) of transvaginally aspirated immature oocytes is an effective and safe assisted reproductive treatment for predicted or high responder patients. Currently, immature oocytes are also being collected from the contralateral ovary during laparoscopy/laparotomy and even ex vivo from the excised ovary or the spent media during ovarian tissue preparation prior to ovarian cortex cryopreservation. The first live births from in vitro-matured ovarian tissue oocytes (OTO-IVM) were reported after monophasic OTO-IVM, showing the ability to achieve mature OTO-IVM oocytes. However, fertilisations rates and further embryological developmental capacity appeared impaired. The introduction of a biphasic IVM, also called capacitation (CAPA)-IVM, has been a significant improvement of the oocytes maturation protocol. However, evidence on OTO-IVM is still scarce and validation of the first results is of utmost importance to confirm reproducibility, including the follow-up of OTO-IVM children. Differences between IVM and OTO-IVM should be well understood to provide realistic expectations to patients.
1. Introduction
2. Current Insight on Oocytes
References
- Edwards, R. Maturation in vitro of human ovarian oocytes. Lancet 1965, 286, 926–929.
- Cross, P.C.; Brinster, R.L. In vitro development of mouse oocytes. Biol. Reprod. 1970, 3, 298–307.
- Wynn, P.; Picton, H.M.; Krapez, J.A.; Rutherford, A.J.; Balen, A.H.; Gosden, R.G. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum. Reprod. 1998, 13, 3132–3138.
- De Vos, M.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. The definition of IVM is clear—Variations need defining. Hum. Reprod. 2016, 31, 2411–2415.
- Pincus, G.; Enzmann, E.V. Can mammalian eggs undergo normal development in vitro? Proc. Natl. Acad. Sci. USA 1934, 20, 121–122.
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 1991, 55, 109–113.
- Trounson, A.; Wood, C.; Kausche, A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil. Steril. 1994, 62, 353–362.
- Shirasawa, H.; Terada, Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod. Med. Biol. 2017, 16, 258–267.
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the development and future of oocyte IVM in clinical practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280.
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; de Vos, M.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157.
- Sánchez, F.; Lolicato, F.; Romero, S.; de Vos, M.; van Ranst, H.; Verheyen, G.; Anckaert, E.; Smitz, J. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum. Reprod. 2017, 32, 2056–2068.
- Vesztergom, D.; Segers, I.; Mostinckx, L.; Blockeel, C.; de Vos, M. Live births after in vitro maturation of oocytes in women who had suffered adnexal torsion and unilateral oophorectomy following conventional ovarian stimulation. J. Assist. Reprod. Genet. 2021, 38, 1–7.
- Siristatidis, C.S.; Maheshwari, A.; Vaidakis, D.; Bhattacharya, S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst. Rev. 2018, 2018, CD006606.
- Child, T.J.; Abdul-Jalil, A.K.; Gulekli, B.; Tan, S.L. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil. Steril. 2001, 76, 936–942.
- Söderström-Anttila, V.; Mäkinen, S.; Tuuri, T.; Suikkari, A.-M. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum. Reprod. 2005, 20, 1534–1540.
- Galvão, A.; Segers, I.; Smitz, J.; Tournaye, H.; de Vos, M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J. Assist. Reprod. Genet. 2018, 35, 2161–2171.
- Chian, R.; Buckett, W.; Tulandi, T.; Tan, S. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum. Reprod. 2000, 15, 165–170.
- Ge, H.-S.; Huang, X.-F.; Zhang, W.; Zhao, J.-Z.; Lin, J.-J.; Zhou, W. Exposure to human chorionic gonadotropin during in vitro maturation does not improve the maturation rate and developmental potential of immature oocytes from patients with polycystic ovary syndrome. Fertil. Steril. 2008, 89, 98–103.
- Holzer, H.E.G.; Scharf, E.; Chian, R.-C.; Demirtas, E.; Buckett, W.; Tan, S.L. In vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertil. Steril. 2007, 88, 62–67.
- Huang, J.Y.; Chian, R.-C.; Tan, S.L. Ovarian hyperstimulation syndrome prevention strategies: In vitro maturation. Semin. Reprod. Med. 2010, 28, 519–531.
- Revel, A.; Safran, A.; Benshushan, A.; Shushan, A.; Laufer, N.; Simon, A. In vitro maturation and fertilization of oocytes from an intact ovary of a surgically treated patient with endometrial carcinoma: Case report. Hum. Reprod. 2004, 19, 1608–1611.
- Segers, I.; Mateizel, I.; van Moer, E.; Smitz, J.; Tournaye, H.; Verheyen, G.; de Vos, M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: A promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J. Assist. Reprod. Genet. 2015, 32, 1221–1231.
- Hourvitz, A.; Yerushalmi, G.; Maman, E.; Raanani, H.; Elizur, S.; Brengauz, M.; Orvieto, R.; Dor, J.; Meirow, D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod. Biomed. Online 2015, 31, 497–505.
- Park, C.W.; Lee, S.H.; Yang, K.M.; Lee, I.H.; Lim, K.T.; Lee, K.H.; Kim, T.J. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin. Exp. Reprod. Med. 2016, 43, 119–125.
- Fasano, G.; Dechène, J.; Antonacci, R.; Biramane, J.; Vannin, A.-S.; van Langendonckt, A.; Devreker, F.; Demeestere, I. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod. Biomed. Online 2017, 34, 575–582.
- Karimi-Zarchi, M.; Mohsenzadeh, M.; Khalili, M.A.; Tabibnejad, N.; Yari, N.; Agha-Rahimi, A. Embryo cryopreservation following in-vitro maturation for fertility preservation in a woman with Mullerian adenosarcoma: A case report. J. Hum. Reprod. Sci. 2017, 10, 138–141.
- Kirillova, A.; Bunyaeva, E.; van Ranst, H.; Khabas, G.; Farmakovskaya, M.; Kamaletdinov, N.; Nazarenko, T.; Abubakirov, A.; Sukhikh, G.; Smitz, J.E.J. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: A study on sibling oocytes. J. Assist. Reprod. Genet. 2021, 38, 1–10.
- Lierman, S.; Tolpe, A.; de Croo, I.; de Gheselle, S.; Defreyne, J.; Baetens, M.; Dheedene, A.; Colman, R.; Menten, B.; T’Sjoen, G.; et al. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertil. Steril. 2021, 116, 1068–1076.
- Anderson, R.A.; McLaughlin, M.; Wallace, W.H.B.; Albertini, D.F.; Telfer, E. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum. Reprod. 2014, 29, 97–106.
- Hambridge, H.L.; Mumford, S.; Mattison, D.; Ye, A.; Pollack, A.; Bloom, M.; Mendola, P.; Lynch, K.L.; Wactawski-Wende, J.; Schisterman, E. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum. Reprod. 2013, 28, 1687–1694.
- Fasano, G.; Moffa, F.; Dechène, J.; Englert, Y.; Demeestere, I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod. Biol. Endocrinol. 2011, 9, 150.
- Revel, A.; Revel-Vilk, S.; Aizenman, E.; Porat-Katz, A.; Safran, A.; Ben-Meir, A.; Weintraub, M.; Shapira, M.; Achache, H.; Laufer, N. At what age can human oocytes be obtained? Fertil. Steril. 2009, 92, 458–463.
- Prasath, E.B.; Chan, M.L.H.; Wong, W.H.W.; Lim, C.J.W.; Tharmalingam, M.D.; Hendricks, M.; Loh, S.F.; Chia, Y.N. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014, 29, 276–278.
- Uzelac, P.S.; Delaney, A.A.; Christensen, G.L.; Bohler, H.C.; Nakajima, S.T. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil. Steril. 2015, 104, 1258–1260.
- Segers, I.; Bardhi, E.; Mateizel, I.; van Moer, E.; Schots, R.; Verheyen, G.; Tournaye, H.; de Vos, M. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum. Reprod. 2020, 35, 2026–2036.
- Sánchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; van Ranst, H.; de Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144.
- Vuong, L.N.; Ho, V.N.A.; Ho, T.M.; Dang, V.Q.; Phung, T.H.; Giang, N.H.; Le, A.H.; Pham, T.D.; Wang, R.; Smitz, J.; et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: A randomized non-inferiority controlled trial. Hum. Reprod. 2020, 35, 2537–2547.
- Strowitzki, T.; Bruckner, T.; Roesner, S. Maternal and neonatal outcome and children’s development after medically assisted reproduction with in-vitro matured oocytes—A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 460–473.





