
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ayobami Adegbite | + 1769 word(s) | 1769 | 2021-09-30 08:10:36 | | | |
| 2 | Catherine Yang | Meta information modification | 1769 | 2021-10-19 03:20:33 | | |
Video Upload Options
Glycoconjugate vaccines have been one tool used to fight against diseases caused by a number of bacteria. Carbohydrates (monosaccharides, oligosaccharides, and polysaccharides) play important functional roles in bacteria. Glycoconjugate vaccines contain oligosaccharides that are attached to a carrier protein.
1. Introduction
Vaccines are preventative measures that aid in controlling the spread of disease in a population [1][2]. For example, this is the second year of the global pandemic caused by SARS-CoV2, and the distribution of vaccines to a critical mass of the population will be crucial to slowing the continuing spread of the coronavirus and decreasing the mortality caused by the disease [3]. Vaccines work by inducing short-lived or long-lasting immune response in humans and animals [4][5]. Vaccines contain antigens that elicit the formation of antibodies toward a particular pathogen through an adaptive immune response [6]. Many types of vaccines exist, including live-attenuated vaccines, inactivated vaccines, subunit, recombinant, and conjugate vaccines [2]. Live-attenuated and inactivated vaccines contain components from the natural pathogen (such as outer membrane vesicles) that can act as self-adjuvants [7]. Adjuvants are non-immunogenic compounds that act to increase the immune response by activating antigen-presenting cells. Other types of vaccines (primarily subunit and recombinant) require the use of adjuvants [7][8]. Glycolipids are one class of adjuvant that has been used. For example, QS-21, an amphipathic saponin has been used as an adjuvant to induce T-cell dependent immune responses in veterinary and human vaccines [9]. Similarly, mimics of phosphatidylinositol mannosides (PIMs) have been studied for their ability to bind to DC-SIGN on dendritic cells [10][11].
Glycoconjugate vaccines have been one tool used to fight against diseases caused by a number of bacteria. Carbohydrates (monosaccharides, oligosaccharides, and polysaccharides) play important functional roles in bacteria. Glycoconjugate vaccines contain oligosaccharides that are attached to a carrier protein. The covalent linkage of polysaccharide to a carrier protein transforms the immune response from a T-cell-independent to a T-cell-dependent response, leading to the production of IgG-based antibody responses [5]. These sugar fragments can be directly isolated from the pathogen or obtained through synthesis. One of the earliest example of a successful glycoconjugate vaccine using pathogen-derived oligosaccharides was Haemophilus influenzae type b [12]. To make this type of vaccine, polysaccharides are obtained from the organism, acid-hydrolyzed to oligosaccharides, and derivatized before conjugation to a derivatized carrier protein [13]. The resulting conjugates are often heterogeneous with random multiple sites of attachment of sugars to protein [14]. Despite this fact, these vaccines are successful in protecting individuals from several different pathogens. Vaccines of the future could be rationally designed to have a defined oligosaccharide chain length and position of conjugation. These types of vaccines will play important roles in defining the relationship between vaccine structure and the strength of the immune response generated.
Chemoenzymatic synthesis is a promising route to obtaining well-defined homogeneous vaccines. Chemical synthesis of oligosaccharides has also been a successful strategy for medically relevant oligosaccharides [15]. In comparison to only chemical synthesis, chemoenzymatic synthesis offers some advantages, such as milder reaction conditions and no need for carbohydrate-protecting groups because of regiospecificity ( Table 1 ). Typically, chemically synthesized substrates are reacted with carbohydrate-producing enzymes under controlled conditions (with a balance of temperature, pH, enzyme concentration, metal ion concentration, acceptor substrate, donor sugar concentration) to produce the desired oligosaccharides [16]. Enzymatic steps could be carried out in tandem or at the same time. One-pot multienzyme synthesis (OPME) approaches involve the combination of many steps in one reaction vessel [16]. Besides the specific enzyme present within the system, recycling enzymes that convert byproducts (free nucleotides) into substrates (nucleotide donor sugars) could also be present to drive efficient oligosaccharide production.
Table 1. Differences of chemoenzymatic and chemical synthesis.
| Chemoenzymatic Synthesis | Chemical Synthesis |
|---|---|
|
|
|
|
2. Chemoenzymatic Synthesis of Meningococcal Oligosaccharides
N. meningitidis is a Gram-negative bacterium and one of the leading causes of bacterial meningitis worldwide. Of the 13 identified serogroups, six have been identified to be pathogenic. The six pathogenic ones are A, B, C, X, W, and Y [17][18][19]. Glycoconjugate vaccines exist for serogroups A, C, W, and Y. A protein-based vaccine is available for serogroup B, and there is no licensed vaccine of either type for serogroup X. There has notably been quite a bit of development in the chemoenzymatic synthesis of meningococcal oligosaccharides ( Figure 1 ), as the enzymes responsible for synthesis in the various serogroups have been characterized by different research groups [20][21][22][23][24][25][26][27][28][29][30].
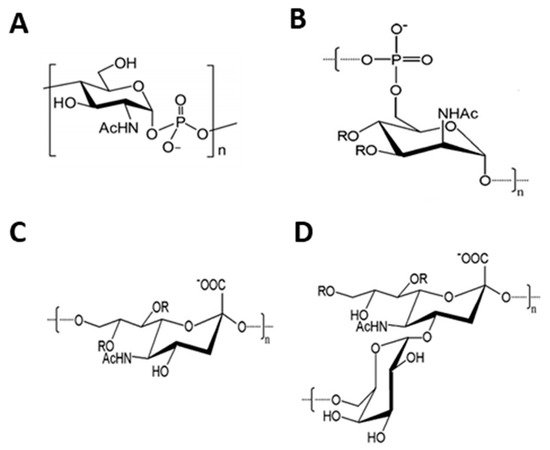
McCarthy et al. used a chemoenzymatic approach to produce a well defined vaccine candidate using click chemistry conjugation [24][31]. A chemically synthesized azido-containing lactoside, 9-azidononanyllactoside, was used as a substrate for Camplyobacter jejuni CSTII sialyltransferase and the N. meningitidis serogroup C polysialytransferase. The inhibitor CMP-9-deoxy-NeuNAc was used to control the chain length of the oligosialic acids produced yielding polymers with median DP25 and DP51, respectively. These oligosialic acids were conjugated to alkyne-modified receptor fragment of the tetanus toxin (TetHc) protein as a carrier. After injection with the glycoconjugates, mice sera showed higher IgG titers over control mice that received unconjugated sialic acids. Antibodies from immunized mice were reactive against serogroup C polysaccharide.
The N. meningitidis serogroup W capsule polymerase synthesizes a heteropolymer of sialic acid α(2,6)-linked to galactose by glycosidic linkage [21][22][30]. This polysaccharide is O -acetylated at positions C7 and C9 [32]. Li et al. synthesized structurally defined synthetic serogroup W oligosaccharides tagged with a hydrophobic chromophore (2-O-(N-benzyloxycarbonyl)aminopropyl α-N-acetylneuraminide) using the capsule polymerase, N. meningitidis CMP-sialic acid synthetase, Streptococcus pneumoniae galactokinase, Bifidobacterium longum UDP-sugar pyrophosphorylase and Pasteurella multocida inorganic pyrophosphatase in a sequential one-pot multienzyme platform [27]. Using this method, defined oligomers of DP > 65 were produced. Overall, the work showed that synthesis of size-controlled oligosaccharides can be produced effectively through modulation of the donor:acceptor ratio. These methods are promising for the development of conjugatable oligosaccharides for vaccine development.
3. Enzymatic Synthesis of Oligosaccharides from Antibiotic-Resistant Bacteria
Antibiotic resistance is a global problem [33]. Bacteria are continually evolving to incorporate mechanisms that weaken the effect of antibiotics. The World Health Organization (WHO) has developed a Global Action Plan to combat antimicrobial resistance [34] The five strategic goals of this plan are: “(1) to improve awareness and understanding of antimicrobial resistance; (2) to strengthen knowledge through surveillance and research; (3) to reduce the incidence of infection; (4) to optimize the use of antimicrobial agents; and (5) to develop the economic case for sustainable investment that takes account of the needs of all countries, and increase investment in new medicines, diagnostic tools, vaccines and other interventions”. Vaccine development is a proactive method to help lower antibiotic resistance. By producing vaccines against antibiotic-resistant pathogens, we can provide crucial protection to a population before they are sickened by disease. The WHO has four levels of priority for pathogens that they are targeting for research and development to prevent antibiotic resistance. These levels are highest priority, critical priority, high priority, and medium priority ( Table 2 ).
Table 2. Types of bacteria that are antibacterial resistant (from WHO).
| WHO Priority Level | Bacteria | Antibiotic Resistance |
|---|---|---|
| Highest | Mycobacterium tuberculosis | Fluoroquinolone |
| Rifampicin | ||
| Isoniazid | ||
| Critical | Acinetobacter baumannii Pseudomonas aeruginosa Enterobacteriaceae spp. |
Carbapenem |
| High | Enterococcus faecium | Vancomycin |
| Staphylococcus aureus | Methicillin, Vancomycin | |
| Helicobacter pylori | Clarithromycin | |
| Campylobacter spp. | Fluoroquinolone | |
| Salmonellae | Fluoroquinolone | |
| Neisseria gonorrhoeae | Cephalosporin, Fluoroquinolone | |
| Medium | Streptococcus pneumoniae | Penicillin |
| Haemophilus influenzae | Ampicillin | |
| Shigella spp. | Fluoroquinolone |
There have been many efforts towards the chemical synthesis of oligosaccharides for homogeneous vaccine development against priority pathogens including Staphylococcus aureus , Streptococcus pneumoniae , Hemophilus influenzae b and Shigella [35][36][37][38][39][40][41]. In this section, we describe some of the advances in the chemoenzymatic/enzymatic synthesis of oligosaccharides from select WHO priority pathogens.
Tuberculosis is caused by the pathogen Mycobacterium tuberculosis . The disease is one of the leading cause of infectious-disease-related death in the world [42]. The only current vaccine againt the pathogen is the BCG live-attenuated vaccine which is believed to be 50% effective and potentially has effectiveness for up to 20 years [43]. In recent work by Li and colleagues, a chemoenzymatic approach was used to facilitate the synthesis of oligosaccharides derived from lipoarabinomannan (LAM) for use in potential vaccine candidates [44]. Lipoarabinomannan and lipomannan are structural components of the M. tuberculosis cell wall and may contribute to antibiotic resistance [45]. Arabinomannan (AM) is what contributes to virulence in LAM and has internal α(1 → 6) and α(1 → 2) mannose covered by a branched arabinan domain. At the non-reducing end of this domain, a common motif of β(1 → 2) arabinose linked to α(1 → 5) and α(1 → 3) aribanose is found. Li et al. synthesized three oligosaccharides containing this motif (ManAra3, containing mannose linked to three arabinose sugars; Man3Ara3, containing three mannose linked to three arabinose sugars; and Man3Ara, containing three mannose linked to arabinose). In their work, a chemoenzymatic approach was developed using enzymatic hydrolysis by a resin-bound lipase B from Candida antarctica (CALB) as a step in synthetic flow chemistry [46][47][48]. This enzyme was able to site-selectively remove acetyl groups from a peracetylated arabinose thioglycoside, which was an important precursor in their synthetic scheme to obtain the ManAra oligosaccharides. The resulting oligosaccharides were conjugated to recombinant human serum albumin (rHSA), and ELISA tests were carried out using serum from patients infected with tuberculosis to determine whether the antibodies present could bind to the synthetic glycoconjugates. Results showed that the ManAra3-rHSA had a higher reaction compared to other glycoconjuates, which may indicate that this motif is a component of the human epitope ( Figure 2 ).
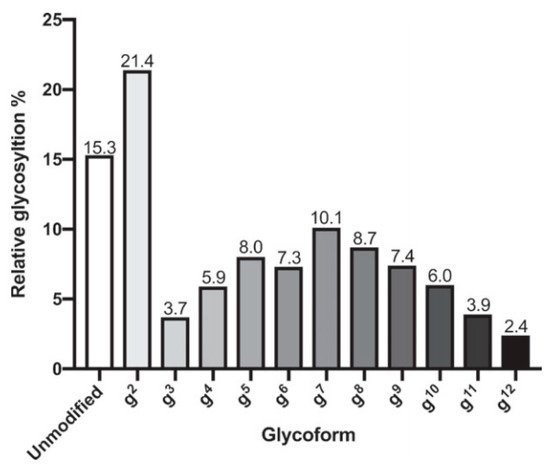
Glycoconjugate formation was confirmed through characterization by NMR, immunoblotting, and mass spectrometry. For the K2-EPA, the most prevalent glycoform was two repeats of the core structure (g 2) ( Figure 3 ). The K1-EPA, K2-EPA, and K1/K2 (bivalent) glycoconjugates produced from these processes were evaluated for their immunogenicity in mice. Serum antibodies from immunized mice induced serotype-specific IgG responses from mice when compared to placebo. The bivalent glycoconjugates produced antibodies against both serotypes. In mice survival studies, the bivalent conjugate increased the survival of mice even at very high challenge of the K1 or K2 serotype dose. This work describes remarkable advances towards the development of a vaccine against hypervirulent Klebsiella . Bioconjugation has also been used for vaccine candidates against Shigella flexneri [49].
References
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of vaccines; health, economic and social perspectives. Front. Microbiol. 2020, 11, 1526.
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287.
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639.
- Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295.
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609.
- Schijns, V.; Fernández-Tejada, A.; Barjaktarović, Ž.; Bouzalas, I.; Brimnes, J.; Chernysh, S.; Gizurarson, S.; Gursel, I.; Jakopin, Ž.; Lawrenz, M.; et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol. Rev. 2020, 296, 169–190.
- Petrovsky, N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015, 38, 1059–1074.
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate immune adjuvants in subunit vaccines. Pharmaceutics 2020, 12, 965.
- Wang, P. Natural and synthetic saponins as vaccine adjuvants. Vaccines 2021, 9, 222.
- Bonam, S.R.; Bhunia, D.; Muller, S.; Nerella, S.G.; Alvala, M.; Mahabalarao, S.K.H. Novel trisaccharide based phospholipids as immunomodulators. Int. Immunopharmacol. 2019, 74, 105684.
- Reina, J.J.; Díaz, I.; Nieto, P.M.; Campillo, N.E.; Páez, J.A.; Tabarani, G.; Fieschi, F.; Rojo, J. Docking, synthesis, and NMR studies of mannosyl trisaccharide ligands for DC-SIGN lectin. Org. Biomol. Chem. 2008, 6, 2743–2754.
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376.
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220.
- Kay, E.; Cuccui, J.; Wren, B.W. Recent advances in the production of recombinant glycoconjugate vaccines. Npj Vaccines 2019, 4, 16.
- Seeberger, P.H.; Werz, D.B. Synthesis and medical applications of oligosaccharides. Nature 2007, 446, 1046–1051.
- Li, W.; McArthur, J.B.; Chen, X. Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res. 2019, 472, 86–97.
- Stephens, D.S. Conquering the meningococcus. FEMS Microbiol. Rev. 2007, 31, 3–14.
- McCarthy, P.C.; Sharyan, A.; Sheikhi Moghaddam, L. Meningococcal vaccines: Current status and emerging strategies. Vaccines 2018, 6, 12.
- Tzeng, Y.L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772.
- Sharyan, A.; Gonzalez, C.; Ukaegbu, O.; Powell, K.; McCarthy, P.C. Determination of the binding affinities of Neisseria meningitidis serogroup w capsule polymerase with two nucleotide sugar substrates. BMC Res. Notes 2018, 11, 482.
- Romanow, A.; Keys, T.G.; Stummeyer, K.; Freiberger, F.; Henrissat, B.; Gerardy-Schahn, R. Dissection of hexosyl- and sialyltransferase domains in the bifunctional capsule polymerases from Neisseria meningitidis w and y defines a new sialyltransferase family. J. Biol. Chem. 2014, 289, 33945–33957.
- Romanow, A.; Haselhorst, T.; Stummeyer, K.; Claus, H.; Bethe, A.; Mühlenhoff, M.; Vogel, U.; von Itzstein, M.; Gerardy-Schahn, R. Biochemical and biophysical characterization of the sialyl-/hexosyltransferase synthesizing the meningococcal serogroup w135 heteropolysaccharide capsule. J. Biol. Chem. 2013, 288, 11718–11730.
- Muindi, K.M.; McCarthy, P.C.; Wang, T.; Vionnet, J.; Battistel, M.; Jankowska, E.; Vann, W.F. Characterization of the meningococcal serogroup x capsule n-acetylglucosamine-1-phosphotransferase. Glycobiology 2014, 24, 139–149.
- Mosley, S.L.; Rancy, P.C.; Peterson, D.C.; Vionnet, J.; Saksena, R.; Vann, W.F. Chemoenzymatic synthesis of conjugatable oligosialic acids. Biocatal. Biotransform. 2010, 28, 41–50.
- Ming, S.A.; Cottman-Thomas, E.; Black, N.C.; Chen, Y.; Veeramachineni, V.; Peterson, D.C.; Chen, X.; Tedaldi, L.M.; Wagner, G.K.; Cai, C.; et al. Interaction of Neisseria meningitidis group x n-acetylglucosamine-1-phosphotransferase with its donor substrate. Glycobiology 2018, 28, 100–107.
- Ming, S.A.; Caro, N.C.; Lanz, N.; Vionnet, J.; Vann, W.F. Effect of acceptor chain length and hydrophobicity on polymerization kinetics of the Neisseria meningitidis group c polysialyltransferase. Biochemistry 2019, 58, 679–686.
- Li, R.; Yu, H.; Muthana, S.M.; Freedberg, D.I.; Chen, X. Size-controlled chemoenzymatic synthesis of homogeneous oligosaccharides of Neisseria meningitidis w capsular polysaccharide. ACS Catal. 2020, 10, 2791–2798.
- Fiebig, T.; Freiberger, F.; Pinto, V.; Romano, M.R.; Black, A.; Litschko, C.; Bethe, A.; Yashunsky, D.; Adamo, R.; Nikolaev, A.; et al. Molecular cloning and functional characterization of components of the capsule biosynthesis complex of Neisseria meningitidis serogroup a: Toward in vitro vaccine production. J. Biol. Chem. 2014, 289, 19395–19407.
- Fiebig, T.; Berti, F.; Freiberger, F.; Pinto, V.; Claus, H.; Romano, M.R.; Proietti, D.; Brogioni, B.; Stummeyer, K.; Berger, M.; et al. Functional expression of the capsule polymerase of Neisseria meningitidis serogroup x: A new perspective for vaccine development. Glycobiology 2014, 24, 150–158.
- Claus, H.; Stummeyer, K.; Batzilla, J.; Mühlenhoff, M.; Vogel, U. Amino acid 310 determines the donor substrate specificity of serogroup w-135 and y capsule polymerases of Neisseria meningitidis. Mol. Microbiol. 2009, 71, 960–971.
- McCarthy, P.C.; Saksena, R.; Peterson, D.C.; Lee, C.H.; An, Y.; Cipollo, J.F.; Vann, W.F. Chemoenzymatic synthesis of immunogenic meningococcal group c polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj. J. 2013, 30, 857–870.
- Li, R.; Kooner, A.S.; Muthana, S.M.; Yuan, Y.; Yu, H.; Chen, X. A chemoenzymatic synthon strategy for synthesizing n-acetyl analogues of o-acetylated N. meningitidis w capsular polysaccharide oligosaccharides. J. Org. Chem. 2020, 85, 16157–16165.
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 2017, 74, 1001–1008.
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015.
- Danieli, E.; Proietti, D.; Brogioni, G.; Romano, M.R.; Cappelletti, E.; Tontini, M.; Berti, F.; Lay, L.; Costantino, P.; Adamo, R. Synthesis of staphylococcus aureus type 5 capsular polysaccharide repeating unit using novel l-fucnac and d-fucnac synthons and immunochemical evaluation. Bioorg. Med. Chem. 2012, 20, 6403–6415.
- Hagen, B.; van Dijk, J.H.M.; Zhang, Q.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Synthesis of the Staphylococcus aureus strain m capsular polysaccharide repeating unit. Org. Lett. 2017, 19, 2514–2517.
- Kaplonek, P.; Khan, N.; Reppe, K.; Schumann, B.; Emmadi, M.; Lisboa, M.P.; Xu, F.F.; Calow, A.D.J.; Parameswarappa, S.G.; Witzenrath, M.; et al. Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc. Natl. Acad. Sci. USA 2018, 115, 13353–13358.
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004, 305, 522–525.
- Hu, Z.; Bongat White, A.F.; Mulard, L.A. Efficient iterative synthesis of o-acetylated tri- to pentadecasaccharides related to the lipopolysaccharide of Shigella flexneri type 3 a through di- and trisaccharide glycosyl donors. Chem. Asian J. 2017, 12, 419–439.
- Mitra, A.; Mukhopadhyay, B. Linear synthesis of the hexasaccharide related to the repeating unit of the o-antigen from Shigella flexneri serotype 1d (i: 7,8). Carbohydr. Res. 2016, 426, 1–8.
- Robbins, J.B.; Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Shiloach, J.; Schneerson, R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei o-specific oligosaccharide-core-protein conjugates. Proc. Natl. Acad. Sci. USA 2009, 106, 7974–7978.
- WHO Global Tuberculosis Report 2020. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020 (accessed on 10 August 2021).
- Moliva, J.I.; Turner, J.; Torrelles, J.B. Prospects in Mycobacterium bovis Bacille Calmette et Guérin (BCG) vaccine diversity and delivery: Why does BCG fail to protect against tuberculosis? Vaccine 2015, 33, 5035–5041.
- Li, Z.; Bavaro, T.; Tengattini, S.; Bernardini, R.; Mattei, M.; Annunziata, F.; Cole, R.B.; Zheng, C.; Sollogoub, M.; Tamborini, L.; et al. Chemoenzymatic synthesis of arabinomannan (am) glycoconjugates as potential vaccines for tuberculosis. Eur. J. Med. Chem. 2020, 204, 112578.
- Abrahams, K.A.; Besra, G.S. Synthesis and recycling of the mycobacterial cell envelope. Curr. Opin. Microbiol. 2021, 60, 58–65.
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420.
- Britton, J.; Jamison, T.F. The assembly and use of continuous flow systems for chemical synthesis. Nat. Protoc. 2017, 12, 2423–2446.
- Panza, M.; Pistorio, S.G.; Stine, K.J.; Demchenko, A.V. Automated chemical oligosaccharide synthesis: Novel approach to traditional challenges. Chem. Rev. 2018, 118, 8105–8150.
- Ravenscroft, N.; Braun, M.; Schneider, J.; Dreyer, A.M.; Wetter, M.; Haeuptle, M.A.; Kemmler, S.; Steffen, M.; Sirena, D.; Herwig, S.; et al. Characterization and immunogenicity of a Shigella flexneri 2a o-antigen bioconjugate vaccine candidate. Glycobiology 2019, 29, 669–680.
- Feldman, M.F.; Bridwell, A.E.M.; Scott, N.E.; Vinogradov, E.; McKee, S.R.; Chavez, S.M.; Twentyman, J.; Stallings, C.L.; Rosen, D.A.; Harding, C.M. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 18655–18663.




