Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Rosaria Plutino | + 6153 word(s) | 6153 | 2021-09-27 08:48:15 | | | |
| 2 | Amina Yu | Meta information modification | 6153 | 2021-09-29 03:09:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Plutino, M.R. Applications of Functional Gold Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/14661 (accessed on 07 February 2026).
Plutino MR. Applications of Functional Gold Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/14661. Accessed February 07, 2026.
Plutino, Maria Rosaria. "Applications of Functional Gold Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/14661 (accessed February 07, 2026).
Plutino, M.R. (2021, September 28). Applications of Functional Gold Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/14661
Plutino, Maria Rosaria. "Applications of Functional Gold Nanoparticles." Encyclopedia. Web. 28 September, 2021.
Copy Citation
Gold nanoparticles were synthesized by Huang et al., Akiyama et al., and Mayya et al., obtaining an average diameter of the particles of about 10–50 nm. Ojea-Jiménez et al., investigating AuNP synthesis, demonstrated the influence of the sequence in the addition of the reagents. In the direct method, an aqueous solution of HAuCl4was heated up to 100 °C for 15 min and, finally, added with sodium citrate.
gold nanoparticles
nanomaterials synthesis
drug delivery
photothermal activity
1. Introduction
Nowadays, nanotechnology and nanochemistry are very often combined in order to develop nanostructured materials and, also, determine to what extent the manipulation of matter on an atomic, molecular, and supramolecular level may affect the desired nanomaterials properties [1]. The atomic structure of materials having nanometric sizes promotes the implementation of their physical, chemical, and biological properties [2]. In particular, the electronic energy levels in nanomaterials are quantized and not continuous as to their corresponding bulk conformation; this effect, known as the quantum confinement effect, demonstrates that material properties are size-dependent [3]. The modification of surface area and electron delimitation, due to the confinement of electronic wave function in up to three physical dimensions, induces the development and the possibility to customize some properties, such as chemical reactivity, melting point, electrical conductivity, fluorescence, and magnetic permeability as a function of the size of nanoparticles [4]. The history of AuNPs dates back to remote times when red ruby glass began to be used; however, they received the maximum attention starting from the end of the seventeenth century. The properties of metallic gold, such as optical and thermal, are explained by describing the plasmon resonance, which makes the AuNPs employable as sensors [5], ultra-small light emitters [6], nano heaters [7], or nano antennas [8]. Gold is an element that has a singular mix of physical and chemical features both in macroscopic and microscopic conditions. While the macroscopic properties concern its unique yellow color, chemical stability, and high redox potential, at the nanometric level, gold features are explained by a combination of the electronic structure with other effects due to the extremely small dimensions. Moreover, this is also due to (i) a high ratio of surface atoms to bulk atoms, (ii) electromagnetic confinement due to a localized plasmon resonance after the interaction with an optical wave, and (iii) the quantum effects, which justify, for instance, the change from metallic to a semiconducting character [9]. One of the most impressive and useful AuNPs properties is plasmon resonance related to the collective behavior of conduction gold electrons. In fact, when it comes to metals, the conduction electrons behave as free charges, which can be excited by an electromagnetic wave. Thus, plasmon waves result from both charge mechanical oscillations and electromagnetic oscillations of the electric field. When this phenomenon occurs at the nanoscale, it is called Localized Surface Plasmon Resonance (LSPR), and it is the result of the confinement of the electric field within a small metallic sphere. This explains the red–purple color of spherical nanoparticles and its slight change when the shape or the surrounding medium are altered. For example, the LSPR is a powerful technique to input energy in metallic nanoparticles, enhancing the light-to-heat conversion. This study reports different chemical and green synthesis methods for the production of gold nanoparticles (AuNPs), namely, chemical reduction or others, such as electrochemical [10], thermal [11], and photochemical reduction techniques. The applications of AuNPs are strictly related to their shape and size; for example, gold nanorods are employed as biosensors, antineoplastic drugs [12], and as carriers in drug delivery systems [13]. AuNPs can penetrate cancer cell membranes, preventing their proliferation and growth [14]. When these nanoparticles interact with light, the oscillating electric field induces the conduction electrons to oscillate with the same frequency of the electromagnetic wave; this is coherent with their plasmon electron cloud and its distribution over the whole nanoparticle volume. The AuNP surface charge is neutralized through undesired aggregation phenomenon. This can be mitigated by using opportune functional capping agents and depositing them on the surface; they can be small molecules, polymers, or biomolecules [15]. Depending on these surface modifications, AuNPs can be employed in engineering, chemical, biochemical, and medical applications [16].
This review collects the synthesis and chemical–physical characterization methods of AuNPs with interesting shapes that are requested in common applications (Figure 1). Their use in the biology and medicine fields are discussed, both for drug delivery and therapeutic treatments. This work concludes with an overview of all AuNP technological applications that could become a part of everyday life in the near future.
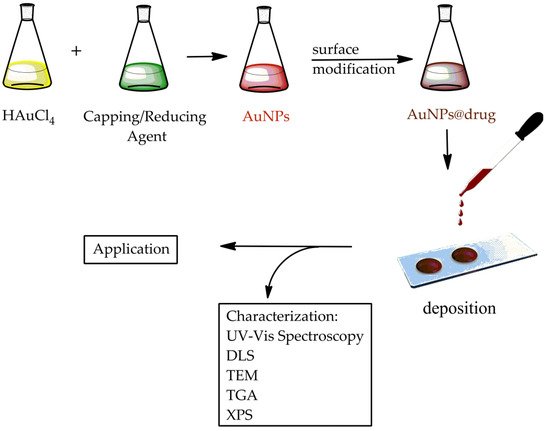
Figure 1. Scheme of the synthesis, characterization, and application of AuNPs.
In particular, AuNPs are employed for medical purposes as:
- -
-
Sensors: AuNPs can be used for protein detection in Raman spectroscopy utilized as support for the implementation of the analysis of vibrational energies of chemical bonds [17].
- -
-
Probes: used for biological imaging application. AuNPs can produce an array of colors employed in dark-field microscopy [18].
- -
-
Diagnostics: AuNPs are able to detect biomarkers as a valid tool in the diagnosis of cancers, infectious agents, and heart diseases [19].
- -
-
Treatment Agent Transport: AuNP surfaces can be functionalized with hundreds of biomolecules, which are delivered to target cells [20].
- -
-
Photodynamic Therapy: AuNPs generate heat when they are irradiated by 700–800-nm wavelengths of light. The heat of these nanoparticles produced when they are inside cancer cells is then exploited to induce death [21].
Most of the aforementioned products are still restricted to the research and development stages, with human tests, delivery systems, and toxicological assessments that have yet to be analyzed and developed.
2. Applications
2.1. Hyperthermia and Photothermal Therapy
Specific optical properties of AuNPs make them powerful nanometric thermal sources, thanks to an intrinsic energy exchange brought to light–heat conversion, generally called the thermo-optical response of nanomaterials. This response is mainly controlled by the behavior of electrons. In stationary conditions, a temperature variation provokes a modification of the material optical index and properties. The thermo-optical response of AuNPs is greatly influenced by the surface electromagnetic field improvement at the SPR [22]. AuNPs can achieve energy by absorbing photons, when exposed to visible incident light, through electron transitions. The relaxation process results in an individual electron–phonon collision and, then, a subsequent energy transfer from electrons to lattice vibrations. The SPR input is a powerful and rapid approach of absorption energy by light beam excitation and the conversion of this energy into heat on a nanoscale. When AuNPs are irradiated with an NIR light beam, the surface electrons are excited and thus they resonate. During electron relaxation, they radiate energy in a nonradiative way, and the surrounding temperature increases. This rise in temperature depends on the AuNP shape and concentration, as well as on their incubation time with tissues and the laser exposure time [23]. The typical absorption spectrum of AuNPs is related to their shape, and the wavelength range is usually between 650 and 900 nm, where the absorption due to tissue is minimal [24]. Gold nanorods have an enhanced length/width ratio, and the absorptive peak of their longitudinal SPR band shifts within the visible and NIR spectrums [25]. Heating could be to release drugs straight into a specific site. Moreover, the AuNP photothermal effect may be used to carry drugs across cell membranes, damage DNA, and produce oxygen-free radicals. Hyperthermia induces the localization of drugs inside a tumor cell by increasing the local blood flow. Furthermore, this condition works at the cellular level by enhancing the permeability and allowing a higher intracellular chemotherapy amount. Photothermal therapy uses NIR light absorption to cause thermal damage [24] by inducing the mechanisms of cellular damage that destroy cancer tissue [26], such as protein denaturation or tissue carbonization. Hyperthermia is based on heating an organ at temperatures between 41 and 45 °C; this therapy can also improve chemotherapy, laser-induced tumor damage [27] and also enhances the photodynamic (PDT) effect [28]. Hyperthermia is an interesting treatment with a lower side-effect profile than conventional cancer therapies (Figure 2). Customized therapy is based on “activated therapy”; in particular, enzyme-cleavable prodrugs are employed [29]. The activated prodrugs release the precursor drug after interacting with a specific biomarker within the cell [30]. Nanotechnology has supported the development of drug delivery systems employed in different clinical applications [31]. Even though there have been different drug delivery nanoparticle- or molecular-based systems all over the scientific bibliography, very few of them have been approved by the Medicines and Healthcare products Regulatory Agency (MHRA), the European Medicines Agency (EMA), or the US FDA, demonstrating difficulty in the clinical application of these nanosystems [32]. For instance, paclitaxel (Abraxane®, Abraxis BioScience Inc., Los Angeles, CA, USA), a 130-nm albumin particle, has been authorized by the US Food And Drug Administration (US FDA) for metastatic breast cancer [33]. Doxorubicin (another Doxil) is another example of an FDA-approved nanoparticle-based drug, which has been validated in metastatic ovarian cancer and AIDS-related Kaposi’s sarcoma therapies. A significant challenge for the implementation of the photothermal therapy effect is the homogeneous distribution of the temperature all over the tissue [34]. Methods that use temperatures above 45 °C to induce irreversible cell damage are related to thermal ablation techniques such as radiofrequency or microwave ablation. This causes a distinct area of cellular apoptosis surrounded by regions receiving less intense hyperthermia. Tumor cells seem to be more sensitive to heat-induced damage than healthy cells. In vivo tests show that tissue depths of approximately 1 cm could be irradiated with NIR light using untargeted AuNPs [35] without visible damage. In particular, the depth of penetration and the selectivity of photothermal therapy are some of the most important challenges for its employment in clinical tests, where tumor tissues may be 5–10 cm deeper. This phenomenon describes the recent research works and applications of AuNPs and their photothermal properties.
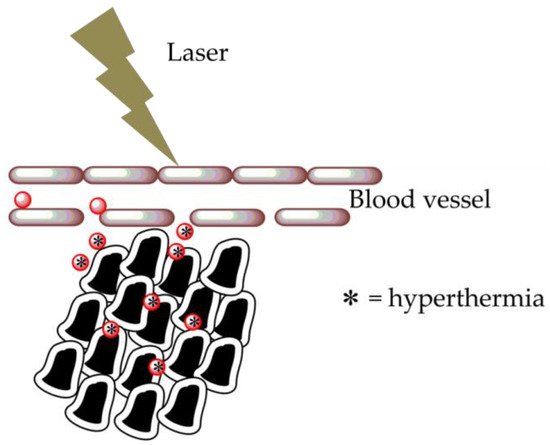
Figure 2. Hyperthermia cancer treatment using AuNPs. Nanoparticles carry a specific binder of the tumor, interacting with abnormal cells due to the implemented permeability of the vessels surrounding the cancer cells. Laser illumination of the AuNPs generates heat production.
2.2. AuNP for Health Applications
The combination of nanoscience and biotechnology has spawned nanobiotechnology; this research area offers a huge opportunity to advance in medical and health treatment, diagnostics, and therapeutics [36]. Among all noble metals, AuNP is the most largely studied thanks to its well-known synthesis procedures and safety profile. AuNP systems are considered a useful tool both in diagnosis and therapy (Theranostics) due to their singular properties, such as penetration and traceability within the body [37].
2.2.1. Biodistribution and Cytotoxicity of AuNP
AuNP biodistribution and toxicity are essentially associated to the way they are introduced into the human body—namely, orally, intravenously, or directly into the target cell. Nanomaterial compositions and sizes are important parameter that regulate the cellular uptake mechanisms, the intracellular localization of AuNP, and their chemical interaction with cells [38]. A study on the influence of the nanoparticle size was carried out, considering the gastrointestinal absorption and the subsequent distribution of AuNPs in the tissue/organ. The latter were administered orally, in in vivo models, and different sizes of nanoparticles were investigated (58, 28, 10, and 4 nm) [39]. The presence of AuNPs in biological samples was qualitatively and quantitatively measured by the TEM analysis. The smallest (4 nm) nanoparticles were found in the kidneys, liver, lungs, spleen, and brain, while the largest (58 nm) AuNPs were mostly detected in the gastrointestinal tract. Through these distribution studies of AuNPs in tissues and organs, the paracellular mechanism suggested that heating could be used to release drugs straight into a specific site without being subjected to organized intracellular destructive processes, such as enzymatic degradation addressed to conjugated proteins or molecular species [40]. Oral and intravenous administrations are based on passive targeting, which, thanks to a greater permeation and retention effect, induces the accumulation of AuNP preferentially in the tumor site [41]. Several studies have shown that the reticuloendothelial system (RES) is the main route of elimination of AuNPs, occurring via macrophages in the liver and spleen. Therefore, the lower the interaction between AuNPs and RES, the higher the blood circulation time, with a consequent increase in intra-tumoral penetration [42]. Another in vivo study on the tissue distribution of different-sized AuNPs administered intravenously tested the presence of gold in different organs and tissues 24 h after injection. It was found that 70–80% of the total injected dose was present in the blood and the liver, regardless of the size of the AuNP [43].
Intra-tumoral administration is a direct method of introducing AuNPs directly into the tumor site [44]. A research study conducted ex vivo on a human eye affected by choroidal melanoma demonstrated the correct distribution of AuNPs within the tumor tissue; on the contrary, no nanoparticles were detected in the extra-tumoral areas [45]. Although this injection technique is able to provide a higher concentration of intra-tumoral AuNPs, resulting in a lower dose to administer, it may be difficult to treat tumors that are not accessible by direct injection [46].
The intracellular responses, the biodistribution, and the cytotoxicity of the nanoparticles depend on several factors, such as the size and shape, surface conjugations, the target cell type, and administration methods [47]. The cytotoxicity data are important to predict the AuNP biocompatibility [48]. Several studies have suggested the dependence of AuNP cytotoxicity on the doses, stabilizing agents employed [49], and target cell type [50]. Nonmalignant cells have been shown to be more sensitive to nanoparticles than cancer cells [51]. Even if a great number of research has shown low AuNP cytotoxicity [52], the wide available literature includes contradictory data because of the diverse cell lines, cell viability assays, the chemical routes employed for AuNP synthesis [53], and the absence of standard safety protocols. Several research studies have been conducted focusing on the relationship between the AuNP properties and cell death mechanisms for different types of tumor cells, and the cellular mechanisms studied are apoptosis, necrosis, and autophagy [54]. It was shown that smaller AuNPs tended to induce more necrosis, and hexagonal ones and nanorods causes more apoptosis, while AuNPs with hydrophobic functions induced greater apoptosis and autophagy than hydrophilic ones.
Another research study summarized the mechanism, the efficacy, and the toxicity of photothermal therapy by using AuNPs of different shapes and sizes [55]. The results showed that smaller AuNP sizes (≤20 nm) have longer blood retention and generate higher heat than larger nanoparticles, which showed a lower toxicity. Moreover, these particles are highly dependent on AuNP surface coating and cellular uptake behavior and cytotoxicity. In the previous paragraphs, different methods of AuNP synthesis were discussed, and in many of these, the surfactants are used as capping agents due to their cytotoxicity and the consequent limited use in clinical applications. To overcome this issue, a surface modification strategy using polymers was implemented and designed [56]. Numerous studies and research have been carried out to develop new AuNP nanosystems for anticancer therapies with potential clinical applications. In fact, the actual chemical use of these nanosystems is not yet applicable due to a series of problems associated with the targeted release of NPs at the tumor site, their biodistribution, and their intrinsic toxicity [57]. The latter is also related to the surface charge and size of AuNPs, with their consequent grouping, and their accumulation in particular biological sites [58]. A research work showed that AuNPs are more likely to accumulate in the liver and spleen, while they have not been detected in the heart, brain, kidneys, lungs, adrenal glands, or mesenteric lymph nodes [59]. However, experimental data are not sufficient to estimate the long-term AuNP cytotoxicity, and further investigations over longer time intervals are necessary.
2.2.2. AuNPs as Delivery Carriers
AuNPs have been used as an excellent system for the delivery of different types of drugs and biomolecules (DNA, RNA, and proteins) to the target sites [60]. The design of an efficient therapy, aimed to release the therapeutic agent, takes place by exploiting both the internal and the external conditions, such as the pH and the presence of oxidizing or reducing agents, and light. The main factor that significantly influences the drug release is the modification and functionalization of the nanocarrier surface [61]. The nonspecific targeting of AuNPs and their ability to stimulate the host’s immune system represent the main limitations in the use of these products as drug delivery systems. To tackle these problems, PEG modifications on the surfaces of AuNPs were carried out, with the aim of protecting the surface and inactivating them. This allowed also to minimize AuNPs’ tendency to stimulate the immune system [62]. In fact, this approach inhibited the adhesion of AuNPs on certain receptors, consequently making them “invisible” to the immune system. However, the specific functionalization of the surface can cause undesired toxic effects. For an effective anticancer therapy, the superficial functionalizations of AuNPs should be customized according to the chemoresistance and the diversity of the genetic makeup of the tumor cells. The efficacy of the anticancer drug transport nanosystems can be implemented by functionalizing the surfaces of the nanoparticles with stromal antagonists. This involves further studying the development of the previously described techniques, particularly with regards to active targeting [63]. Several biomolecules such as oligonucleotides, proteins, and peptides have been tested for targeted delivery to target cells using AuNP as the nanocarrier. In this regard, gene therapy has been found to be a highly efficient method for treating genetically acquired diseases, but it has also shown safety problems due to the random immune response and cytotoxicity [62]. For this reason, nonviral gene delivery systems were considered. An effective drug delivery system should allow facilitated entry into the cell, the protection of the nucleic acid from degradation by the nucleases, and the subsequent release of the nucleic acid in a functional form within the nucleus and the therapeutic effects of releasing all types of oligonucleotides, such as single-stranded or double-stranded DNA, plasmids, and single-stranded RNA [64]. Nucleic acid strands can be chemically modified with thiol groups to bind them to AuNPs covalently. It has been shown that AuNPs possess a high-affinity constant for the nucleotide sequence, showing a 99% higher cell internalization without causing cytotoxic effects, being also resistant to enzymatic degradation. Nucleic acids of an anionic nature can interact electrostatically with cationic AuNPs; in particular, a system of functionalized AuNPs with amino acids was created for the release of DNA, whose gene expression was much more efficient than the covalent functionalization of AuNPs [65]. Some studies have shown how AuNPs can identify the surface of an ionic protein through a complementary electrostatic interaction, limiting its activity [66]. For example, AuNPs functionalized and stabilized with chitosan can transport and release insulin, with a decrease in blood glucose levels after 2 h of oral administration [67]. These studies showed how functionalization can improve the efficiency and specificity of the nanoparticles in the target organ/tissue, revealing their potential use in nanopharmacology and nanomedicine [68]. The release of large biomolecules targeted on specific cells requires a prior step of cellular internalization before their release. Therefore, various factors need to be taken into account before using them for the delivery of biomolecules, such as the sizes and shapes of AuNPs, their functionalization, and their biodistribution and retention. A parameter that affects a drug’s release at the target site is the pH [69]. In particular, tumor cells have pH values ranging from 5.7 to 7.8. This condition causes both the breakdown of the bond sensitive to acids and variations of the total charge due to protonation and morphological alterations of the transported biomolecules [70]. Glutathione-mediated drug release is an alternative nonenzymatic approach for activating prodrugs in the intracellular environment. The underlying mechanism is the osmotic one, which exploits the difference in the glutathione concentration in the intracellular (1–10 mM) and extracellular matrix (2 μM) [71]. These methods focus on the formation of a disulfide bridge between drugs and their carrier. Along with the potential effectiveness of this approach, the modification of the reactivity conditions of the disulfide bond are challenging, mainly because of the collateral exchange reactions in the presence of cysteines localized on the surfaces of the blood proteins. This, in fact, causes the formation of different protein derivatives—transporters with different bioaccumulation and pharmacokinetic profiles.
2.3. Diagnostics
There is little research aimed at the direct use of AuNPs for cancer diagnostics and therapy [63] and even fewer technologies based on gold nanoparticles approved by the FDA for diagnostic and therapeutic purposes in medicine [72]. One of the clinical studies conducted by Astra Zeneca in collaboration with Cytimmune is mainly focused on the use of AuNPs for tumor therapy. Aurimune (CYT-6091) was used as a vehicle to transport recombinant human tumor necrosis factor alpha (rhTNF) into tumors, which allowed chemotherapy to enter cancer cells, damaging them. Thanks to the ability of AuNPs to absorb NIR light, the interest in photothermal conversion, selective targeting of tumor cells, and in vivo biodistribution of AuNPs has been growing [44]. The absorption of light causes a localized increase in temperature, resulting in the thermal dissolution of solid tumors [73]. An imaging technology has recently been developed during the focal ablation of prostate tumor tissue through direct laser irradiation from nanoparticles. This is the only ultra-focal tumor ablation therapy designed to implement therapy efficacy with minimal side effects. A recent clinical study based on AuNPs aimed to evaluate the feasibility of a new method used in oncology for the identification of gastric diseases based on the analysis of breaths with an array of nanosensors. The latter may be able to provide a noninvasive screening tool that distinguishes tumors located in the gastrointestinal tract from related precancerous lesions [74] and provides a diagnosis of pulmonary arterial hypertension.
2.3.1. Enhanced Permeability and Retention Effect (EPR) and Tumor Targeting
The enhanced permeability and retention (EPR) effect supports a clarification for the specific targeting of AuNPs in the tumor cells [75]. As a consequence of tumor physiology, AuNPs selectively accumulate inside solid tumor tissues that are made of leaky blood vessels, with junction gaps varying the dimension from 100 nm to 780 nm [76], instead of normal capillaries, which have about 20-nm pore diameters [77]. Different research works have demonstrated that AuNPs up to 100 nm in size can pass through the reticuloendothelial system (RES) to accumulate in tumor tissues and be retained inside [78]. This is a passive method to convey AuNPs into tumor cells in order to irradiate them by photothermal therapy. This approach may be suitable for tumors less than 3 cm in size [79], but the most considerable restrain is the extensive biological heterogeneity of tumors and, therefore, the bio-specificity deficiency. Tumor tissues characterized by a poor vascularization, like prostate or pancreatic cancer, may not accumulate AuNPs only via the EPR effect. To increase the AuNP concentration inside tumor cells, active targeting has consequently been investigated by bonding a targeting side that is overexpressed in cancer cells [80]. Two different targeting mechanisms are employed to promote tumor specificity. AuNPs conjugated to a specific receptor are delivered through the typical mechanism for that particular receptor [81]. The most serious disadvantage related to active targeting is the dimensions of AuNPs that inhibits their transport across bio-barriers [82].
2.3.2. Application of AuNPs for Small Molecule Detection
AuNPs can be employed as the Solid-Phase Extraction (SPE) adsorbent [83] as efficient sensors [84] for metal cation enhancement and revelation. For instance, dithiocarbamate functionalized diethanolamine (DEA) was employed to alter the AuNP surface, improving its affinity. The DEA is a symmetric compound used to chelate cations [85]. The synthesized DEA@AuNPs exhibit an adequate selectivity towards lead ions, depending on the coordination of the N and O DEA atoms, with the Pb2+ cations building a framework. AuNPs have been also used for the detection of environmental pollutants, such as polycyclic aromatic hydrocarbons (PAHs), based on the powerful affinity adsorption among the AuNP unmodified surfaces and PAHs: their determination was conducted by means of laser-excited time-resolved Shpol’skii spectrometry [86]. Furthermore, sixteen PAHs have been analyzed by using GC-MS with the support of AuNP-based extraction [87]. Some studies have connected AuNP-based nanoextraction with mass spectrometric detection [88]. AuNPs have been investigated for application in laser desorption ionization (LDI) mass spectrometry due to the high surface area and laser light absorbance, easy sample preparation, and analytical procedures [89]. Surface-modified and non-surface-modified nanoparticles have been utilized for detecting small molecules and ions, such as Hg2+ cations [90], amino thiols [88], and mono- and disaccharides [89]. The amount of detection was determined by a TOF MS analysis [90]. The results showed that unmodified AuNPs exhibit a stronger trapping efficiency for neutral carbohydrates and higher ionization efficiencies compared to capped AuNPs. Thanks to the great affinity of AuNPs towards thiol functional groups, AuNPs were employed for aminothiol compound extraction. AuNP detection was achieved by different methods, such as fluorescence detection [91] and capillary electrophoresis [92]. Hybrid materials of AuNPs with other materials have increasingly gained attention, especially for sensing with target specificity. For instance, a composite realized by mixing hybrid AuNPs and reduced graphene oxide has been tested as an adsorbing agent for the purification of mycotoxins and their HPLC-MS identification [93]. Polydopamine-stabilized magnetic AuNPs [94] have been synthesized for the detection of steroid hormones in milk, urine, and water samples. AuNPs were dispersed in an ionic solution of imidazolium functional group compounds prepared with the addition of pyridoxine (vitamin B6) and folic acid (vitamin B9) from the biological samples. The results of the HPLC-UV analysis showed a high selectivity, good extraction, and limit of detection [95]. The amphiphilic nature of the ionic solution enhanced the stability of the colloidal-modified AuNPs [96].
2.3.3. Application of AuNPs for Detection of Biological Molecules
The most used device, developed for the detection of biological molecules such as proteins, hormones, and pesticides, is based on a polymeric membrane sheet where the sample is analyzed through lateral diffusion. The AuNP surface is modified with specific antibodies for selective detection exploiting an immune reaction. Once the target molecules sample is poured on the polymeric membrane sheet, the antibodies move together with the mobile phase, binding their corresponding antigen. This bond inhibits antibodies to bind the antigen, linked on a test line membrane, covalently. If the sample solution does not contain antigen molecules, the antibody sites are free to bind with the test line membrane, and the consequent accumulation of AuNPs causes the test line color to change; an increase in the amount of antigens corresponds to a decrease in the amount of antibodies bonded to the test line, thus resulting in the color fading. This method is employed to detect and characterize compounds such as hormones, pesticides, or drugs [97]. The sensitivity of the test is improved by adjusting the number of antigens linked to the AuNPs [98]. For the quantification and revelation of large proteins, a direct method is used: two different antibodies, both having a high affinity to the protein, are respectively bound to AuNPs and the line test. The target molecules bind to both antibodies, inducing the development of a colored line, whereas, in the absence of a protein, there is no binding, and no color change is observed [99]. The principle described for protein quantification is also used to develop devices for the rapid detection of polynucleotides [100]. The sensitivity of these devices may be improved by fixing an enzyme to the AuNP surfaces, and they can have different applications, as they are easy to use, compact, portable, and cost-effective.
2.4. Imaging
Several imaging techniques exploit the surface plasmon resonance effect characteristic of AuNP. Larger nanoparticles (400 nm) can be detected using an optical microscope in the phase contrast mode, which involves only scattered light in dark-field microscopy. Small AuNPs can only absorb light, causing the local heating of the environment, which can be detected by photothermal imaging, by fluorescence microcopy, which allows for single particle level detection, by multiphoton SPR microscopy, etc. Immunostaining is a TEM imaging technique based on AuNP conjugated with antibodies that bind fixed and permeabilized cell antigens [42]. The field of the research and development of innovative and highly efficient AuNP-based contrast agents for magnetic resonance imaging (MRI) is rapidly growing. The sensitivity of MRIs can be optimized by using AuNP as a carrier of gadolinium chelate models, currently utilized in the clinical diagnosis field. The core–shell particles of magnetite/AuNP employed in imaging have been synthesized thanks to the magnetic features of iron oxide (Fe3O4) and the optical properties of AuNP [101]. AuNP-assisted MRI could also be potentially used as a probe sensitive towards different types of proteins. Among the spectroscopic methods that characterize the electromagnetic field resulting from the plasmon resonance of AuNP surfaces, surface-enhanced Raman scattering (SERS) is the preferred one, since it allows a net enhancement of the signal and a limit of detection at the single-molecule level. The Raman effect in molecules far from the surface of an AuNP is weak, since the visible light not absorbed by these molecules is not dispersed in an anelastic way [102]. The intensity of the Raman signals on the AuNP surface is very high, because it is directly proportional to the fourth power of the local electric field, implemented thanks to the surface plasmon resonance and the charge transfer between the AuNP metal surface and the adsorbed molecules. The interference of the molecules that contribute to adsorption can prevent the detection of target molecules. The plasmon band moves from the visible region for spherical AuNPs to the NIR by changing the size, shape, and level of aggregation [103].
2.5. Application of AuNPs for the Biomarker Analysis
Biomarkers are exceptional and valuable indicators of a specific disorder. However, their analysis requires efficient sample preparation for a good selective extraction that needs to be suitable for the sensitive detection techniques [104]. Oxidized phospholipids are used as biomarkers for cardiovascular diseases, but an accurate analysis is not yet available. Haller et al. studied an AuNP nanoextraction method for trapping oxidized phospholipids through chemical identification by a bifunctional compound containing a hydrazide group for trapping phospholipid carbonyl groups and a thiol functional group for the selective bonding of AuNP derivatives. After the transamination of hydroxylamine, the oxime derivative of carbonylated phospholipids was analyzed by HPLC-ESI-MS/MS [105]. In this case study, different derivatization and releasing agents, used in different concentrations, were explored to develop an optimized sample preparation process for achieving strong selective enrichment and sensitive revelation. The correlation of the AuNP derivatives with the MALDI-TOF-MS analysis has recently shown significant improvement in sample homogeneity, decreasing the sample preparation time and removing the matrix ion interferences [106]. For instance, AuNPs functionalized with an aminooxy group were used for the chemical enrichment of glycosphingolipids (GSLs) in the living cell surface, and their identification was carried out by SPR and the MALDI-TOF-MS analysis. The AuNP trapping was found to be dependent of the ozonolysis reaction, which led to the formation of an oxime. Laser irradiation induced the oxime bond break and imino alcohol ion release for the MS analysis. Sudhir et al. reported the biomarker analysis for peptide and protein detection [107]. The results showed that the hydrophilic peptide methionine–encephalin and leucine–encephalin extractions were dependent on the AuNP surface charge and the target peptides’ isoelectric points (pI). The maximum extraction efficiencies were yielded above the peptides pI thanks to good ion pairing conditions at the considered pH value. Furthermore, unmodified AuNPs were also employed. For instance, Faccenda et al. studied unmodified AuNPs for the isolation of peptides containing a thiol group from the proteolysis of S-nitrosated proteins. The detection and characterization of the S-nitrosylation sites in the protein were carried out by MALDI-TOF [108]. In another study, bi-functionalized AuNPs were put together with a multivalent carbohydrate and a photoreactive site for the affinity extraction of carbohydrate-binding proteins, which were analyzed by the MALDI-TOF-MS analysis and fluorescence imaging after release by adding 2-mercaptoethanol [109]. This method allowed the simultaneous purification and characterization of carbohydrate-binding proteins. Combining functional groups could facilitate sample preparation, which is important for the analysis of low-abundance biomolecules, without interference and improve the sensitivity. For example, AuNP was functionalized with anti-insulin for trapping insulin in biofluid samples [110]. This characterization is very important, because it is one of the most meaningful post-translational protein modifications involved in different biological processes [111]. The high surface-to-volume ratio of AuNPs contributes to modifying the surface with different ligands to achieve the goal of affinity glycoprotein extraction and enrichment by suitable surface modification [112]. Tran et al. reported the synthesis of ultrasmall AuNPs, functionalized by hydrazide, which were used for the extraction and enrichment of N-glycosylated peptides. In this procedure, the AuNPs were modified with glutathione, and afterwards, the carboxylic acid groups were derivatives with hydrazine to obtain AuNPs@hydrazide. The extraction and enrichment procedure of the N-glycosylated peptides was realized after the reaction of aldehyde groups of carbohydrates with hydrazine groups on AuNP surfaces. The analysis was performed by HPLC and QTOF mass spectrometer [113]. Likewise, AuNPs functionalized with boronic acid exhibited a specific recognition of the glycan compounds, depending on the reversible covalent bonds between the acid and cis-diol groups. These AuNPs showed significant selectivity for the glycopeptides [114]. The AuNPs were also immobilized on monoliths to achieve enhanced surface reactive sites [112]. For instance, AuNPs grafted on poly(glycidylmethacrylate-co-poly (ethylene glycol) diacrylate) monoliths were functionalized with cysteine. Since grafting implemented the reactive sites and enhanced the hydrophilicity, this system was used for the efficient and selective enrichment of glycopeptides by hydrophilic interaction chromatography. The glycopeptides or deglycosylated peptides were analyzed by MALDI-TOF-MS and Nano RPLC-ESI-MS/MS [115]. AuNPs hybridized with different nanomaterials could improve the excellent properties of both materials and increase the application sphere [116].
2.6. Application of AuNPs as Bio-Barcodes
Bio-barcodes are employed to quickly identify very low amounts of various proteins by using a series of reactions for the (i) specific detection, (ii) transcription, and (iii) amplification of the signal. The first reaction involves recognition of the target protein by binding specific antibodies to a magnetic substrate, even though the free proteins are washed away (Figure3a). During the second reaction step, AuNPs carrying specific antibodies and oligonucleotides are both added; the antibodies are bound to allow the specific link of AuNPs to the proteins restrained by the magnetic substrate (Figure3b). The transcription step involves the binding of AuNPs with an oligonucleotide linked to the chip, which is complementary to the sequence of antibodies fixed on the AuNP surfaces (Figure 3c). The last reaction consists of the reaction of Ag(I) with the AuNP surfaces in the presence of reducing agents, such as hydroquinone, resulting in the reduction and deposition of Ag(I) in the metallic nanoparticles (Figure 3d) for amplification (Figure 3e) of the signal related to the AuNPs [117]. This procedure promoted the amplification of the signal by increasing the AuNP sizes. To detect different proteins simultaneously, a two-dimensional array of oligonucleotides was used. Each oligonucleotide sequence corresponded to a specific antibody, so various changes of the protocols [118] approved the development of chips for bio-barcodes with a colorimetric reading or fluorescent biosensor [119].
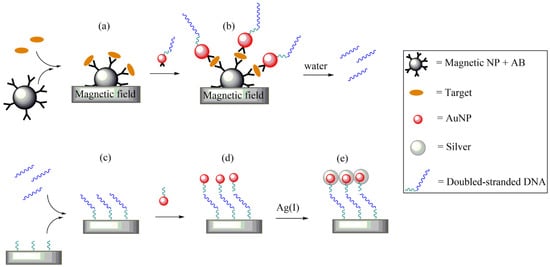
Figure 3. Functioning scheme of the AuNPs used in a bio-barcode. (a) Complex formed by a magnetic nanoparticle carrying specific antibodies and a target molecule, attracted on a magnetic substrate. (b) AuNPs bearing a double-stranded DNA and an antibody. (c) The double-stranded DNA washed away by water moves and interacts with a polynucleotide fixed to a chip. (d) Added AuNPs are functionalized by a complementary polynucleotide. (e) Finally, silver deposition allows the amplification of the detection signal.
3. Conclusions
This review described in detail the studies and experimental results concerning AuNPs. In particular, the paper described different types of synthesis and functionalization methods, as well as various characterization techniques and possible biomedical applications, concerning AuNPs. The prospects for studying AuNPs are very promising; their synthesis can be done by different methods with no toxic effects, obtaining singular optical, physicochemical, and biological features. AuNPs present great potential in the modern biomedical field, and this review collected different synthesis methodologies that are used in the reduction of gold ions into metallic gold and the consequent functionalizations. In this paper, the most-used characterization methods to determine the sizes of AuNPs and their eventual functionalization are also illustrated. AuNPs also require a further stabilization analysis in biological fluids for in vitro and in vivo testing. The applications of functionalized AuNPs in medicine and biotechnology have highly developed recently. The latest research works, conducted under different experimental conditions and protocols, show conflicting results. The relative toxicity of AuNPs is still the subject of scientific research. Accurate therapeutic dosages, the delivery mechanism, and the absence of a toxicity database need to be discussed before the usage of nanocarriers in clinical trials. Antitumor-targeted drug delivery and biological marker systems are among the most important application fields. Metallic nanoparticles—in particular, AuNPs—have achieved great attention because of their size-dependent features and biological behavior, improved biocompatibility, stability, and oxidation resistance. AuNPs are appropriate systems for targeted and controlled drug delivery or even to enhance the external treatment potential. AuNPs are suitable agents for drug delivery systems because of the tenability of nanoparticle surfaces with various molecules, such as amino acids and peptides, oligonucleotides, antibodies, etc., to facilitate the loading of a drug. Drug delivery systems in which AuNPs behave as carriers represent an interesting application that requires more investigation to overcome the limitations and to improve the effectiveness and efficiency of drug release at the desired site. Gold has different chemical and physical properties, such as a high electronegativity, its tendency to link with other gold atoms, and its variable colors of dispersed colloids. These features are related to its electronic structure, and they explain the resulting suitability for its many applications. This review focused on the principles of thermo-optical properties emerging from plasmon resonance, as well as on some possible applications in biology, drug delivery, and therapy.
References
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346.
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740.
- Čitaković, N. Physical Properties of Nanomaterials. Vojnotehnički Glasnik 2019, 67, 159–171.
- Caballero-Calero, O.; D’Agosta, R. Review—Towards the Next Generation of Thermoelectric Materials: Tailoring Electronic and Phononic Properties of Nanomaterials. ECS J. Solid State Sci. Technol. 2017, 6, N3065–N3079.
- Barattucci, A.; Plutino, M.R.; Faggi, C.; Bonaccorsi, P.; Monsù Scolaro, L.; Aversa, M.C. Mono- and trinuclear tripodal platinum(II) chelated complexes containing a pyridine/sulfoxide based anchoring framework. Eur. J. Inorg. Chem. 2013, 2013, 3412–3420.
- Ray, M.; Basu, T.S.; Bandyopadhyay, N.R.; Klie, R.F.; Ghosh, S.; Raja, S.O.; Dasgupta, A.K. Highly lattice-mismatched semiconductor-metal hybrid nanostructures: Gold nanoparticle encapsulated luminescent silicon quantum dots. Nanoscale 2014, 6, 2201–2210.
- Chen, L.; Si, L.S.; Wu, F.; Ying Chan, S.; Yu, P.; Fei, B. Electrical and mechanical self-healing membrane using gold nanoparticles as localized “nano-heaters”. J. Mater. Chem. C 2016, 4, 10018–10025.
- Kucherik, A.; Kutrovskaya, S.; Osipov, A.; Gerke, M.; Chestnov, I.; Arakelian, S. Nano-Antennas Based on Silicon-Gold Nanostructures. Sci. Rep. 2019, 9, 1–6.
- Kaminker, R.; Lahav, M.; Motiei, L.; Vartanian, M.; Popovitz-Biro, R.; Iron, M.A.; van der Boom, M.E. Molecular Structure-Function Relations of the Optical Properties and Dimensions of Gold Nanoparticle Assemblies. Angew. Chem. 2010, 122, 1240–1243.
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141.
- Wostek-Wojciechowska, D.; Jeszka, J.K.; Uznanski, P.; Amiens, C.; Chaudret, B.; Lecante, P. Synthesis of gold nanoparticles in solid state by thermal decomposition of an organometallic precursor. Mater. Sci. Pol. 2004, 22, 407–413.
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120.
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248.
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elem. Med. Biol. 2017, 40, 10–23.
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol-gel treatment of textiles for the entrapping of an antioxidant/anti-inflammatory molecule: Functional coating morphological characterization and drug release evaluation. Appl. Sci. 2020, 10, 2287.
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684.
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New gold nanostructures for sensor applications: A review. Materials 2014, 7, 5169–5201.
- Ma, J.; Liu, Y.; Gao, P.F.; Zou, H.Y.; Huang, C.Z. Precision improvement in dark-field microscopy imaging by using gold nanoparticles as an internal reference: A combined theoretical and experimental study. Nanoscale 2016, 8, 8729–8736.
- Cordeiro, M.; Carlos, F.F.; Pedrosa, P.; Lopez, A.; Baptista, P.V. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics 2016, 6, 43.
- Raliya, R.; Saha, D.; Chadha, T.S.; Raman, B.; Biswas, P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci. Rep. 2017, 7, 1–8.
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552.
- Rashidi-Huyeh, M.; Palpant, B. Counterintuitive thermo-optical response of metal-dielectric nanocomposite materials as a result of local electromagnetic field enhancement. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 1–8.
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Tech. 2011, 74, 592–604.
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124.
- Ahmad, M.Z.; Akhter, S.; Rahman, Z.; Akhter, S.; Anwar, M.; Mallik, N.; Ahmad, F.J. Nanometric gold in cancer nanotechnology: Current status and future prospect. J. Pharm. Pharmacol. 2013, 65, 634–651.
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113–121.
- Milane, L.; Ganesh, S.; Shah, S.; Duan, Z.F.; Amiji, M. Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J. Control. Release 2011, 155, 237–247.
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094.
- Huang, X.; Swierczewska, M.; Choi, K.Y.; Zhu, L.; Bhirde, A.; Park, J.; Kim, K.; Xie, J.; Niu, G.; Lee, K.C.; et al. Multiplex imaging of an intracellular proteolytic cascade by using a broad-spectrum nanoquencher. Angew. Chem. Int. Ed. 2012, 51, 1625–1630.
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Nanotheranostics Pers. Med. 2016, 13, 1–337.
- Cafeo, G.; Carbotti, G.; Cuzzola, A.; Fabbi, M.; Ferrini, S.; Kohnke, F.H.; Papanikolaou, G.; Plutino, M.R.; Rosano, C.; White, A.J.P. Drug delivery with a calixpyrrole-trans-Pt(II) complex. J. Am. Chem. Soc. 2013, 135, 2544–2551.
- Anderson, N.L. The clinical plasma proteome: A survey of clinical assays for proteins in plasma and serum. Clin. Chem. 2010, 56, 177–185.
- Retèl, V.P.; Hummel, M.J.M.; van Harten, W.H. Review on early technology assessments of nanotechnologies in oncology. Mol. Oncol. 2009, 3, 394–401.
- Levi-Polyachenko, N.H.; Stewart IV, J.H. Clinical relevance of nanoparticle induced hyperthermia for drug delivery and treatment of abdominal cancers. Open Nanomed. J. 2011, 3, 24–37.
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554.
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996.
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 1–17.
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20.
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936.
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015, 5, 40514–40520.
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2010, 60, 977–985.
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794.
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919.
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118.
- Akintelu, S.A.; Yao, B.; Folorunso, A.S. Green Synthesis, Characterization, and Antibacterial Investigation of Synthesized Gold Nanoparticles (AuNPs) from Garcinia kola Pulp Extract. Plasmonics 2021, 16, 157–165.
- Kanavi, M.R.; Asadi, S.; Ahmadieh, H. Ex vivo distribution of gold nanoparticles in choroidal melanoma. Int. J. Nanomed. 2017, 12, 8527–8529.
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of 11 Metal Oxide Nanoparticles to Three Mammalian Cell Types In Vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929.
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016, 20, 1–11.
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782.
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119.
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256.
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int. J. Mol. Sci. 2018, 19, 754.
- Asadi, S.; Bianchi, L.; De Landro, M.; Korganbayev, S.; Schena, E.; Saccomandi, P. Laser-induced optothermal response of gold nanoparticles: From a physical viewpoint to cancer treatment application. J. Biophotonics 2021, 14, e202000161.
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217.
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393.
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theoretical analysis at different settings. Int. J. Hyperth. 2017, 33, 150–159.
- Phillips, W.T.; Bao, A.; Brenner, A.J.; Goins, B.A. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv. Drug Deliv. Rev. 2014, 76, 39–59.
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384.
- Mandal, T.K.; Fleming, M.S.; Walt, D.R. Preparation of Polymer Coated Gold Nanoparticles by Surface-Confined Living Radical Polymerization at Ambient Temperature. Nano Lett. 2002, 2, 3–7.
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54.
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Front. Immunol. 2019, 10, 1998.
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857.
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979.
- Zhao, X.; Huang, Q.; Jin, Y. Gold nanorod delivery of LSD1 siRNA induces human mesenchymal stem cell differentiation. Mater. Sci. Eng. C 2015, 54, 142–149.
- Hou, Z.; Wang, Z.; Liu, R.; Li, H.; Zhang, Z.; Su, T.; Yang, J.; Liu, H. The effect of phospho-peptide on the stability of gold nanoparticles and drug delivery. J. Nanobiotechnol. 2019, 17, 88.
- Verma, A.; Simard, J.M.; Worrall, J.W.E.; Rotello, V.M. Tunable Reactivation of Nanoparticle-Inhibited β-Galactosidase by Glutathione at Intracellular Concentrations. J. Am. Chem. Soc. 2004, 126, 13987–13991.
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426.
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205.
- Gao, W.; Chan, J.M.; Farokhzad, O.C. reviews pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920.
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692.
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29.
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2021, 1–16.
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802.
- Kommareddy, S.; Tiwari, S.B.; Amiji, M.M. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol. Cancer Res. Treat. 2005, 4, 615–625.
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Jain, R.K.; Torchilin, V.P. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995, 55, 3752–3756.
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915.
- Levine, E.A.; Stewart IV, J.H.; Russell, G.B.; Geisinger, K.R.; Loggie, B.L.; Shen, P. Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy for Peritoneal Surface Malignancy: Experience with 501 Procedures. J. Am. Coll. Surg. 2007, 204, 943–953.
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028.
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; et al. Erratum: Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7785.
- Altintas, I.; Kok, R.J.; Schiffelers, R.M. Targeting epidermal growth factor receptor in tumors: From conventional monoclonal antibodies via heavy chain-only antibodies to nanobodies. Eur. J. Pharm. Sci. 2012, 45, 399–407.
- Karimipour, G.; Ghaedi, M.; Sahraei, R.; Daneshfar, A.; Biyareh, M.N. Modification of gold nanoparticle loaded on activated carbon with bis(4-methoxysalicylaldehyde)-1,2-phenylenediamine as new sorbent for enrichment of some metal ions. Biol. Trace Elem. Res. 2012, 145, 109–117.
- Priyadarshini, E.; Pradhan, N.; Panda, P.K.; Mishra, B.K. Biogenic unmodified gold nanoparticles for selective and quantitative detection of cerium using UV-vis spectroscopy and photon correlation spectroscopy (DLS). Biosens. Bioelectron. 2015, 68, 598–603.
- Kiriakidou, K.; Plutino, M.R.; Prestopino, F.; Monari, M.; Johansson, M.; Elding, L.I.; Valls, E.; Gobetto, R.; Aime, S.; Nordlander, E. Detection of a novel intermediate in the addition of thiols to osmium carbonyl clusters. Chem. Commun. 1998, 1, 2721–2722.
- Wang, H.; Campiglia, A.D. Direct determination of benzopyrene in water samples by a gold nanoparticle-based solid phase extraction method and laser-excited time-resolved Shpol’skii spectrometry. Talanta 2010, 83, 233–240.
- Wilson, W.B.; Hewitt, U.; Miller, M.; Campiglia, A.D. Water analysis of the sixteen environmental protection agency-polycyclic aromatic hydrocarbons via solid-phase nanoextraction-gas chromatography/mass spectrometry. J. Chromatogr. A 2014, 1345, 1–8.
- Huang, Y.F.; Chang, H.T. Nile red-adsorbed gold nanoparticle matrixes for determining aminothiols through surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2006, 78, 1485–1493.
- Su, C.L.; Tseng, W.L. Gold nanoparticles as assisted matrix for determining neutral small carbohydrates through laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2007, 79, 1626–1633.
- Lin, Y.W.; Chen, W.T.; Chang, H.T. Exploring the interactions between gold nanoparticles and analytes through surface-assisted laser desorption/ ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 933–938.
- Lin, J.H.; Chang, C.W.; Tseng, W.L. Fluorescent sensing of homocysteine in urine: Using fluorosurfactant-capped gold nanoparticles and o-Phthaldialdehyde. Analyst 2010, 135, 104–110.
- Shen, C.C.; Tseng, W.L.; Hsieh, M.M. Selective enrichment of aminothiols using polysorbate 20-capped gold nanoparticles followed by capillary electrophoresis with laser-induced fluorescence. J. Chromatogr. A 2009, 1216, 288–293.
- Jiang, K.; Huang, Q.; Fan, K.; Wu, L.; Nie, D.; Guo, W.; Wu, Y.; Han, Z. Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018, 264, 218–225.
- Liu, S.; Lämmerhofer, M. Functionalized gold nanoparticles for sample preparation: A review. Electrophoresis 2019, 40, 2438–2461.
- Zare, F.; Ghaedi, M.; Daneshfar, A. Application of an ionic-liquid combined with ultrasonic-assisted dispersion ofgold nanoparticles for micro-solid phase extraction of unmetabolized pyridoxine and folic acid in biological fluids prior to high-performance liquid chromatography. RSC Adv. 2015, 5, 70064–70072.
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 20016.
- Delmulle, B.S.; De Saeger, S.M.D.G.; Sibanda, L.; Barna-Vetro, I.; Van Peteghem, C.H. Development of an immunoassay-based lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J. Agric. Food Chem. 2005, 53, 3364–3368.
- Aveyard, J.; Nolan, P.; Wilson, R. Improving the sensitivity of immunoassays by tuning gold nanoparticles to the tipping point. Anal. Chem. 2008, 80, 6001–6005.
- Fernández-Sánchez, C.; McNeil, C.J.; Rawson, K.; Nilsson, O.; Leung, H.Y.; Gnanapragasam, V. One-step immunostrip test for the simultaneous detection of free and total prostate specific antigen in serum. J. Immunol. Methods 2005, 307, 1–12.
- Suzuki, T.; Tanaka, M.; Otani, S.; Matsuura, S.; Sakaguchi, Y.; Nishimura, T.; Ishizaka, A.; Hasegawa, N. New rapid detection test with a combination of polymerase chain reaction and immunochromatographic assay for Mycobacterium tuberculosis complex. Diagn. Microbiol. Infect. Dis. 2006, 56, 275–280.
- Zhang, Y.; Yang, H.; Zhou, Z.; Huang, K.; Yang, S.; Han, G. Recent Advances on Magnetic Relaxation Switching Assay-Based Nanosensors. Bioconjug. Chem. 2017, 28, 869–879.
- Blanco-Formoso, M.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Fabrication and SERS properties of complex and organized nanoparticle plasmonic clusters stable in solution. Nanoscale 2020, 12, 14948–14956.
- Wang, Z.; Huo, Y.; Ning, T.; Liu, R.; Zha, Z.; Shafi, M.; Li, C.; Li, S.; Xing, K.; Zhang, R.; et al. Composite Structure Based on Gold-Nanoparticle Layer and HMM for Surface-Enhanced Raman Spectroscopy Analysis. Nanomaterials 2021, 11, 587.
- Hinterwirth, H.; Stübiger, G.; Lindner, W.; Lämmerhofer, M. Gold nanoparticle-conjugated anti-oxidized low-density lipoprotein antibodies for targeted lipidomics of oxidative stress biomarkers. Anal. Chem. 2013, 85, 8376–8384.
- Haller, E.; Stübiger, G.; Lafitte, D.; Lindner, W.; Lämmerhofer, M. Chemical recognition of oxidation-specific epitopes in low-density lipoproteins by a nanoparticle based concept for trapping, enrichment, and liquid chromatography-tandem mass spectrometry analysis of oxidative stress biomarkers. Anal. Chem. 2014, 86, 9954–9961.
- Nagahori, N.; Abe, M.; Nishimura, S.I. Structural and functional glycosphingolipidomics by glycoblotting with an aminooxy-functionalized gold nanoparticle. Biochemistry 2009, 48, 583–594.
- Sudhir, P.R.; Wu, H.F.; Zhou, Z.C. Identification of peptides using gold nanoparticle-assisted single-drop microextraction coupled with AP-MALDI mass spectrometry. Anal. Chem. 2005, 77, 7380–7385.
- Faccenda, A.; Bonham, C.A.; Vacratsis, P.O.; Zhang, X.; Mutus, B. Gold nanoparticle enrichment method for identifying S-nitrosylation and S-glutathionylation sites in proteins. J. Am. Chem. Soc. 2010, 132, 11392–11394.
- Sakurai, K.; Hatai, Y.; Okada, A. Gold nanoparticle-based multivalent carbohydrate probes: Selective photoaffinity labeling of carbohydrate-binding proteins. Chem. Sci. 2016, 7, 702–705.
- Liang, K.; Wu, H.; Li, Y. Immune-enrichment of insulin in bio-fluids on gold-nanoparticle decorated target plate and in situ detection by MALDI MS. Clin. Proteom. 2017, 14, 1–9.
- Rudd, P.M.; Wormald, M.R.; Dwek, R.A. Glycosylation and the immune system. J. Protein Chem. 1998, 17, 519.
- Alwael, H.; Connolly, D.; Clarke, P.; Thompson, R.; Twamley, B.; O’Connor, B.; Paull, B. Pipette-tip selective extraction of glycoproteins with lectin modified gold nano-particles on a polymer monolithic phase. Analyst 2011, 136, 2619–2628.
- Tran, T.H.; Park, S.; Lee, H.; Park, S.; Kim, B.; Kim, O.H.; Oh, B.C.; Lee, D.; Lee, H. Ultrasmall gold nanoparticles for highly specific isolation/enrichment of N-linked glycosylated peptides. Analyst 2012, 137, 991–998.
- Yao, G.; Zhang, H.; Deng, C.; Lu, H.; Zhang, X.; Yang, P. Facile synthesis of 4-mercaptophenylboronic acid functionalized gold nanoparticles for selective enrichment of glycopeptides. Rapid Commun. Mass Spectrom. 2009, 23, 3493–3500.
- Liang, Y.; Wu, C.; Zhao, Q.; Wu, Q.; Jiang, B.; Weng, Y.; Liang, Z.; Zhang, L.; Zhang, Y. Gold nanoparticles immobilized hydrophilic monoliths with variable functional modification for highly selective enrichment and on-line deglycosylation of glycopeptides. Anal. Chim. Acta 2015, 900, 83–89.
- Dhanyalayam, D.; Scrivano, L.; Parisi, O.I.; Sinicropi, M.S.; Fazio, A.; Saturnino, C.; Plutino, M.R.; Di Cristo, F.; Puoci, F.; Cappello, A.R.; et al. Biopolymeric self-assembled nanoparticles for enhanced antibacterial activity of Ag-based compounds. Int. J. Pharm. 2017, 517, 395–402.
- Taton, T.A.; Mirkin, C.A.; Letsinger, R.L. Scanometric DNA array detection with nanoparticle probes. Science 2000, 289, 1757–1760.
- Nam, J.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive. Science 2003, 301, 1884–1887.
- Goluch, E.D.; Nam, J.M.; Georganopoulou, D.G.; Chiesl, T.N.; Shaikh, K.A.; Ryu, K.S.; Barron, A.E.; Mirkin, C.A.; Liu, C. A bio-barcode assay for on-chip attomolar-sensitivity protein detection. Lab Chip 2006, 6, 1293–1299.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




