
Video Upload Options
Cold Atmospheric Plasma (CAP) is a near-room-temperature partially ionized gas, composed of reactive oxygen and nitrogen species. CAP also generates physical factors, including ultraviolet irradiation, thermal emission, and an electromagnetic (EM) effect. The multimodal chemical and physical nature of CAP makes it a suitable, controllable, flexible, and even a self-adaptive tool for many medical and biological applications, ranging from microorganism sterilization, dermatitis, wound healing, and cancer therapy. It is promising that CAP could help to mitigate the COVID 19 pandemic by effectively inactivating the SARS-CoV-2 virus on diverse surfaces.
Biological killing is a foundation to understand these applications. Reactive species and their radical effects are the foundation to cause the CAP-based biological destruction in most cases. Basically, plasma medicine has even been regarded as a reactive species-based medicine. Here, we provide a systematic introduction and critical summary of the entire picture of biological killing due to CAP treatment and corresponding mechanisms based on the latest discoveries. This work provides guiding principles for diverse applications of CAP in modern biotechnology and medicine.
1. CAP and Plasma Medicine
CAP is a near-room-temperature ionized gas composed of products including neutral particles, such as neutral atoms and molecules; charged particles, such as ions; electrons; and diverse, long-lived and short-lived reactive species, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) [1]. CAP is also referred to as nonthermal plasma (NTP), cold plasma, physical plasma, and gas plasma in many references [2]. CAP is a non-equilibrium plasma in which heavy particles have effective temperatures close to room temperature through weak elastic collisions during the discharge process [3]. CAP also generates several physical effects, including thermal effect, UV effect, and EM effect [4].
Three types of CAP sources have been widely used in plasma medicine and can be roughly divided into three categories: direct discharge sources, indirect discharge sources, and hybrid discharge sources [5]. Despite different morphologies, power input, and reactive species generation in CAP, their chemical composition and physical effects are quite similar. CAP can be precisely controlled by modulating basic operational parameters (gas flow rate, etc.) and discharge parameters (such as discharge voltage, current, duty circle, etc.) [6].
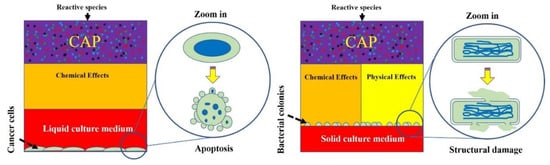
Plasma generated by these sources can be used to directly touch biological samples, which exposes samples to reactive species and physical factors. Alternatively, biological adaptive solutions, such as medium and phosphate-buffered saline (PBS), can be used as a carrier of these long-lived reactive species to exert a killing effect on viruses and cells [7]. Reactive species will cause oxidative stress in the CAP-treated cells and trigger cell-death pathways if the reactive species’ dose on a single cell is sufficiently large [8]. The biological effects of physical factors have been hypothesized for a long time but without clear evidence until very recently. One possible candidate is the EM effect from CAP jet, which causes structural damage on melanoma cells and triggers quick necrosis [9].
The multimodal chemical and physical nature of CAP makes it a suitable, controllable, flexible, and even a self-adaptive tool for many medical applications, ranging from microorganism sterilization, dermatitis, wound healing, and cancer therapy. For microorganism sterilization, particularly bacterial inactivation, CAP can cause strong damage on both gram-positive and gram-negative bacteria, including some multi-drug resistant bacteria [10]. The biofilm composed of a complex microorganism community can also be effectively inactivated by CAP treatment [11]. These anti-bacterial capacities of CAP may be a foundation to drastically improve wound healing efficacy [12]. Over the past decade, CAP has shown impressive potential as a novel anti-cancer tool both in vitro and in vivo. CAP can selectively kill many cancer cell lines while having only limited side effects on normal counteracting cell lines [13]. Importantly, a simple CAP treatment just on the skin above the subcutaneous tumor site could effectively decrease the tumor size and extend life in mice, which demonstrates the non-invasive potential of CAP as a novel anti-cancer modality [14]. Besides, the inactivation of viruses by CAP has also been reported in many studies and has recently been summarized [15]. The CAP-triggered cell death or virus inactivation is the foundation of nearly all these applications (Figure 1).
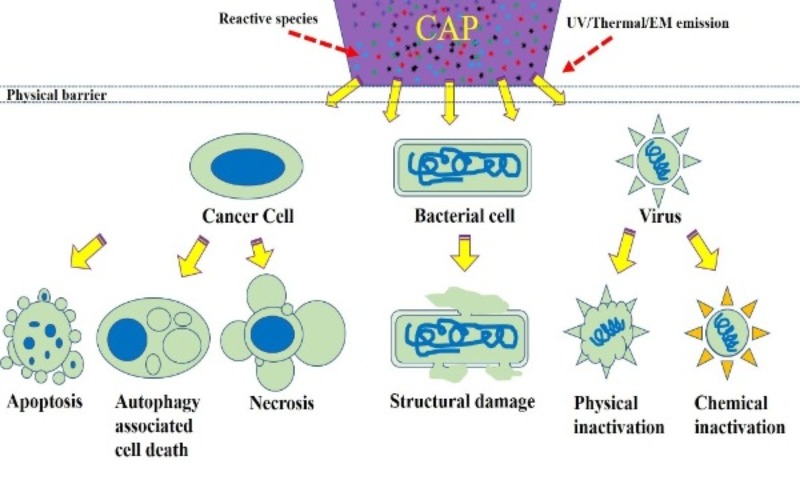 Figure 1. A schematic illustration of biological killing by CAP.
Figure 1. A schematic illustration of biological killing by CAP.
2. Cross-Species Similarities of Biological Killing
References
- A V Phelps; Zoran Petrović; Cold-cathode discharges and breakdown in argon: surface and gas phase production of secondary electrons. Plasma Sources Science and Technology 1998, 8, R21-R44, 10.1088/0963-0252/8/3/201.
- Gillian E Conway; Alan Casey; Vladimir Milosavljevic; Yupeng Liu; Orla Howe; Pj Cullen; James F Curtin; Non-thermal atmospheric plasma induces ROS-independent cell death in U373MG glioma cells and augments the cytotoxicity of temozolomide. British Journal of Cancer 2016, 114, 435-443, 10.1038/bjc.2016.12.
- Michael Keidar; A prospectus on innovations in the plasma treatment of cancer. Physics of Plasmas 2018, 25, 083504, 10.1063/1.5034355.
- M. Laroussi; X. Lu; M. Keidar; Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. Journal of Applied Physics 2017, 122, 020901, 10.1063/1.4993710.
- Thomas VON Woedtke; Anke Schmidt; Sander Bekeschus; Kristian Wende; Klaus-Dieter Weltmann; Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011-1026, 10.21873/invivo.11570.
- Dayun Yan; Li Lin; Wenjun Xu; Niku Nourmohammadi; Jonathan H Sherman; Michael Keidar; Cold plasma-based control of the activation of pancreatic adenocarcinoma cells. Journal of Physics D: Applied Physics 2019, 52, 445202, 10.1088/1361-6463/ab36d4.
- Dayun Yan; Jonathan H. Sherman; Michael Keidar; The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anti-Cancer Agents in Medicinal Chemistry 2018, 18, 769-775, 10.2174/1871520617666170731115233.
- David B Graves; The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics 2012, 45, 263001–42, 10.1088/0022-3727/45/26/263001.
- Dayun Yan; Qihui Wang; Manish Adhikari; Alisa Malyavko; Li Lin; Denis B. Zolotukhin; Xiaoliang Yao; Megan Kirschner; Jonathan H. Sherman; Michael Keidar; et al. A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment. ACS Applied Materials & Interfaces 2020, 12, 34548-34563, 10.1021/acsami.0c06500.
- Georg Daeschlein; Matthias Napp; Stine Lutze; Andreas Arnold; Sebastian Von Podewils; Denis Guembel; Michael Jünger; Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2015, 13, 143-149, 10.1111/ddg.12559.
- Dana Ziuzina; Daniela Boehm; Sonal Patil; Pj Cullen; Paula Bourke; Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLOS ONE 2015, 10, e0138209, 10.1371/journal.pone.0138209.
- G. Isbary; G. Morfill; H.U. Schmidt; M. Georgi; K. Ramrath; J. Heinlin; S. Karrer; M. Landthaler; T. Shimizu; B. Steffes; et al.W. BunkR. MonettiJ.L. ZimmermannR. PomplW. Stolz A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. British Journal of Dermatology 2010, 163, 78-82, 10.1111/j.1365-2133.2010.09744.x.
- Dayun Yan; Annie Talbot; Niki Nourmohammadi; Jonathan H. Sherman; Xiaoqian Cheng; Michael Keidar; Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins (Review). Biointerphases 2015, 10, 040801, 10.1116/1.4938020.
- Marc Vandamme; Eric Robert; Stéphanie Lerondel; Vanessa Sarron; Delphine Ries; Sébastien Dozias; Julien Sobilo; David Gosset; Claudine Kieda; Brigitte Legrain; et al.Jean-Michel PouvesleAlain Le Pape ROS implication in a new antitumor strategy based on non-thermal plasma. International Journal of Cancer 2011, 130, 2185-2194, 10.1002/ijc.26252.
- Arijana Filipić; Ion Gutierrez-Aguirre; Gregor Primc; Miran Mozetič; David Dobnik; Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends in Biotechnology 2020, 38, 1278-1291, 10.1016/j.tibtech.2020.04.003.
- Gregory Fridman; Alexander Fridman; Alexander F Gutsol; Victor Vasilets; Gary D Friedman; Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric Pressure Plasma on Bacteria and Mechanisms of Such Interaction. 2007 IEEE 34th International Conference on Plasma Science (ICOPS) 2007, 4, 322-322, 10.1109/ppps.2007.4345628.
- Li Guo; Ruobing Xu; Lu Gou; Zhichao Liu; Yiming Zhao; Dingxin Liu; Lei Zhang; Hailan Chen; Michael Kong; Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Applied and Environmental Microbiology 2018, 84, e00726, 10.1128/aem.00726-18.
- Dayun Yan; Haitao Cui; Wei Zhu; Niki Nourmohammadi; Julian Milberg; Lijie G. Zhang; Jonathan H. Sherman; Michael Keidar; The Specific Vulnerabilities of Cancer Cells to the Cold Atmospheric Plasma-Stimulated Solutions. Scientific Reports 2017, 7, 4479, 10.1038/s41598-017-04770-x.
- Fariba Saadati; Hamed Mahdikia; Hojjat-Allah Abbaszadeh; Mohammad-Amin Abdollahifar; Maryam Sadt Khoramgah; Babak Shokri; Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Scientific Reports 2018, 8, 7689, 10.1038/s41598-018-25990-9.
- Dayun Yan; Annie Talbot; Niki Nourmohammadi; Xiaoqian Cheng; Jerome Canady; Jonathan H Sherman; Michael Keidar; Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Scientific Reports 2015, 5, 18339, 10.1038/srep18339.
- Dayun Yan; Haitao Cui; Wei Zhu; Annie Talbot; Lijie Grace Zhang; Jonathan H. Sherman; Michael Keidar; The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma. Scientific Reports 2017, 7, 10831.
- Dayun Yan; Li Lin; Wenjun Xu; Niku Nourmohammadi; Jonathan H. Sherman; Michael Keidar; Universality of Micromolar-Level Cell-Based Hydrogen Peroxide Generation during Direct Cold Atmospheric Plasma Treatment. Plasma Medicine 2017, 8, 335-343, 10.1615/plasmamed.2018028781.
- Zahra Nasri; Giuliana Bruno; Sander Bekeschus; Klaus-Dieter Weltmann; Thomas von Woedtke; Kristian Wende; Development of an electrochemical sensor for in-situ monitoring of reactive species produced by cold physical plasma. Sensors and Actuators B: Chemical 2020, 326, 129007, 10.1016/j.snb.2020.129007.
- Dayun Yan; Wenjun Xu; Xiaoliang Yao; Li Lin; Jonathan H. Sherman; Michael Keidar; The Cell Activation Phenomena in the Cold Atmospheric Plasma Cancer Treatment. Scientific Reports 2018, 8, 15418, 10.1038/s41598-018-33914-w.
- Dayun Yan; Qihui Wang; Alisa Malyavko; Denis B. Zolotukhin; Manish Adhikari; Jonathan H. Sherman; Michael Keidar; The anti-glioblastoma effect of cold atmospheric plasma treatment: physical pathway v.s. chemical pathway. Scientific Reports 2020, 10, 11788, 10.1038/s41598-020-68585-z.
- Angela Privat Maldonado; Deborah O’Connell; Emma Welch; Roddy Vann; Marjan W. Van Der Woude; Spatial Dependence of DNA Damage in Bacteria due to Low-Temperature Plasma Application as Assessed at the Single Cell Level. Scientific Reports 2016, 6, 35646, 10.1038/srep35646.
- Hak Jun Ahn; Kang Il Kim; Geunyoung Kim; Eunpyo Moon; Sang Sik Yang; Jong-Soo Lee; Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLOS ONE 2011, 6, e28154, 10.1371/journal.pone.0028154.
- Hak Jun Ahn; Kang Il Kim; Nguyen Ngoc Hoan; Churl Ho Kim; Eunpyo Moon; Kyeong Sook Choi; Sang Sik Yang; Jong-Soo Lee; Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLOS ONE 2014, 9, e86173, 10.1371/journal.pone.0086173.
- Oleg Lunov; Vitalii Zablotskii; Olexander Churpita; Ales Jäger; Leoš Polívka; Eva Syková; Alexandr Dejneka; Sarka Kubinova; The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2015, 82, 71-83, 10.1016/j.biomaterials.2015.12.027.




