Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshihiro Kita | + 975 word(s) | 975 | 2021-08-31 13:28:08 | | | |
| 2 | Camila Xu | Meta information modification | 975 | 2021-09-24 03:37:54 | | | | |
| 3 | Camila Xu | Meta information modification | 975 | 2021-09-24 03:38:15 | | | | |
| 4 | Conner Chen | Meta information modification | 975 | 2021-10-12 10:53:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kita, T. Adrenomedullin. Encyclopedia. Available online: https://encyclopedia.pub/entry/14492 (accessed on 01 March 2026).
Kita T. Adrenomedullin. Encyclopedia. Available at: https://encyclopedia.pub/entry/14492. Accessed March 01, 2026.
Kita, Toshihiro. "Adrenomedullin" Encyclopedia, https://encyclopedia.pub/entry/14492 (accessed March 01, 2026).
Kita, T. (2021, September 24). Adrenomedullin. In Encyclopedia. https://encyclopedia.pub/entry/14492
Kita, Toshihiro. "Adrenomedullin." Encyclopedia. Web. 24 September, 2021.
Copy Citation
Adrenomedullin (AM) is a bioactive peptide with various physiological functions, including vasodilation, angiogenesis, anti-inflammation, organ protection, and tissue repair.
adrenomedullin
inflammatory bowel disease
ulcerative colitis
Crohn’s disease
1. Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are the most common types of chronic inflammatory bowel disease (IBD). The number of people with IBD worldwide reached over 6.8 million in 2017 [1]. The cause of IBD is unknown but is thought to be caused by abnormalities in intestinal immunity, a genetic predisposition, and environmental factors, such as diet and intestinal microflora.
Although there is no cure for IBD, there have been remarkable therapeutic developments that contribute to the induction and maintenance of remission over the past 20 years, including anti-TNF-alpha antibodies, tacrolimus, anti-IL-12/23p40 antibodies, Janus kinase (JAK) inhibitors, and integrin inhibitors. While these drugs have shown excellent efficacy, they also negatively affect patients’ immune systems, leading some patients to develop infections and malignant lymphomas.
Severe infections such as tuberculosis and Pneumocystis pneumonia require serious attention, particularly in elderly patients and patients with underlying diseases, such as diabetes mellitus. Further, the above treatments demonstrate a diminishing therapeutic response, as they are immunogenic and targets of anti-drug antibodies. Therefore, the development of new therapeutic agents for IBD requires efficacy, safety, and immunogenicity.
Adrenomedullin (AM), an endogenous vasodilatory peptide, was isolated from human pheochromocytoma by Kitamura et al. in 1993 [2]. AM was initially studied as a circulatory agonist but was later found to promote angiogenesis, organ protection, and anti-inflammatory immune activity. AM is also widely expressed in the gastrointestinal tract epithelia and effectively treats gastric ulcers and enteritis in animal models. Therefore, we began translational research on the clinical application of AM in IBD [3].
2. Structure and Biosynthesis of Adrenomedullin
AM is composed of 52 amino acids [2] and has a ring structure consisting of six amino acids and a C-terminal amide structure (Figure 1). These two structural features are essential for their biological activity. It also shares homology with calcitonin gene-related peptide (CGRP), amylin, and adrenomedullin 2/intermedin (AM2/IMD) to form the calcitonin peptide superfamily.
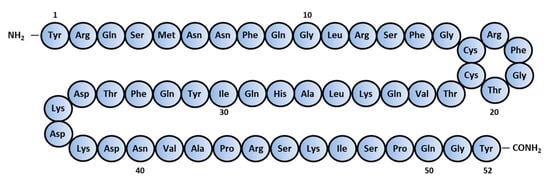
Figure 1. Amino-acid sequence of human Adrenomedullin.
AM is widely expressed throughout the blood vessels, heart, lungs, kidneys, and gastrointestinal tract and is highly concentrated in the adrenal medulla. The pro-adrenomedullin N-terminal 20 peptide (PAMP) has a shorter duration of antihypertensive activity than AM and cooperatively regulates blood circulation with AM (Figure 2) [4]. Although mid regional pro-adrenomedullin (MR-pro ADM) has no biological activity, it has been attracting attention as a biomarker for the prognosis of heart failure [5], myocardial infarction [5], community-acquired pneumonia [6], septic shock [7], and COVID-19 [8][9].
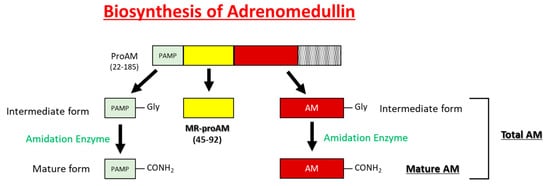
Figure 2. Schematic representations of the processing of AM, MR-proAM, and PAMP from proAM.
3. Adrenomedullin Receptors
The AM receptor consists of a complex of calcitonin receptor-like receptors (CRLR; a seven-transmembrane G protein receptor) and a receptor activity-modifying protein (RAMP; a single transmembrane receptor). There are three subtypes of RAMPs, RAMP1, RAMP2, and RAMP3, and the CRLR/RAMP1 complex has a high affinity for CGRP, while the CRLR/RAMP2 and CRLR/RAMP3 complexes have high affinity for AM. The CRLR/RAMP2 complex comprises the AM1 receptor, and the CRLR/RAMP3 complex constitutes the AM2 receptor (Figure 3).
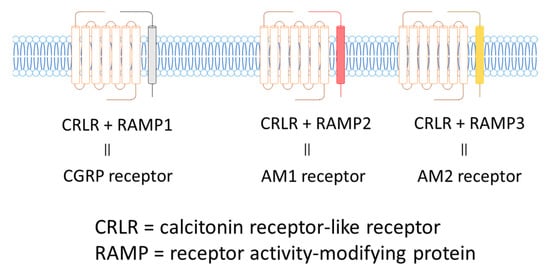
Figure 3. CGRP and AM Receptors.
AM contributes to the development and homeostasis of blood vessels and lymphatic vessels; AM knockout mice develop defective embryos due to defective blood and lymphatic vessel formation. Furthermore, studies using RAMP2 and RAMP3 gene knockout mice report that the AM1 receptor is involved in angiogenesis and vascular homeostasis, while the AM2 receptor regulates lymph vessel function [10]. Thus, we speculate that AM influences vascular and lymphatic system ecological functions through two receptors.
4. General Physiological Effects of Adrenomedullin
AM circulates the blood constitutively and is detected in healthy individuals; its expression is enhanced by mechanical stimuli, such as:
-
Myocardial and vascular wall stretching
-
Organ ischemia and hypoxia
-
Inflammatory factors, such as inflammatory cytokines, angiotensin II, oxidative stress, and various tissue stress.
AM promotes various pathophysiological effects, such as (Table 1) [11]:
Table 1. Diverse physiological effects of adrenomedullin.
| Vasodilation |
| Angiogenesis |
| Cardioprotection |
| Nephroprotection |
| Anti-oxidation |
| Anti-apoptosis |
| Tissue repair and regeneration |
5. Anti-Inflammatory Effects of Adrenomedullin
Blood (AM) is markedly elevated during severe inflammation, such as burns, pancreatitis, and systemic inflammatory response syndrome (SIRS) associated with sepsis [12].
In vitro studies using cultured vascular smooth muscle cells (VSMCs) and endothelial cells have shown that pro-inflammatory cytokines such as IL-1, tumor necrosis factor (TNF)-α, and lipopolysaccharide (LPS) stimulate AM expression in smooth muscle and endothelial cells [15]. In addition, the monocyte/macrophage cell line (RAW 264.7), murine celiac macrophages, and peripheral blood-derived monocytes have shown that monocyte-macrophage differentiation enhances AM production. Further, AM inhibits monocyte and macrophage TNF-α and IL-6 secretion following LPS-stimulation [16]. In an in vivo mouse study of sepsis treated with LPS and D-galactosamine, AM-overexpressing mice had demonstrated milder liver failure than wild-type mice, demonstrating that endogenous AM protects against SIRS [17]. Further, septic mice models treated with AM demonstrated improved hemodynamics and decreased pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [18] than control mice. Thus, AM ameliorates inflammatory conditions and is expected to be applied clinically to treat various inflammatory diseases.
6. Physiological Functions of Adrenomedullin in the Gastrointestinal Tract
AM is widely expressed throughout the mucosal epithelium, glandular duct cells, neuroendocrine cells, and smooth muscle cells of the gastrointestinal tract, between the oral cavity and the large intestine [19]. The physiological effects of AM include suppressed gastric acid secretion via somatostatin in the stomach, enhanced electrolyte secretion in the colon, suppressed gastrointestinal motility, and changes to microcirculation flux. Additionally, AM has similar physiological and antibacterial effects as defensins and may contribute to the mucosal defense system by regulating the oral and intestinal microbiome [20].
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30.
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560.
- Ashizuka, S.; Inatsu, H.; Inagaki-Ohara, K.; Kita, T.; Kitamura, K. Adrenomedullin as a potential therapeutic agent for inflammatory bowel disease. Curr. Protein Pept. Sci. 2013, 14, 246–255.
- Tsuruda, T.; Kato, J.; Kuwasako, K.; Kitamura, K. Adrenomedullin: Continuing to Explore Cardioprotection. Peptides 2019, 111, 47–54.
- Self, W.H.; Storrow, A.B.; Hartmann, O.; Barrett, T.W.; Fermann, G.J.; Maisel, A.S.; Struck, J.; Bergmann, A.; Collins, S.P. Plasma Bioactive Adrenomedullin as a Prognostic Biomarker in Acute Heart Failure. Am. J. Emerg. Med. 2016, 34, 257–262.
- Liu, D.; Xie, L.; Zhao, H.; Liu, X.; Cao, J. Prognostic Value of Mid-Regional Pro-Adrenomedullin (MR-ProADM) in Patients with Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2016, 16, 232.
- Elke, G.; Bloos, F.; Wilson, D.C.; Brunkhorst, F.M.; Briegel, J.; Reinhart, K.; Loeffler, M.; Kluge, S.; Nierhaus, A.; Jaschinski, U.; et al. The Use of Mid-Regional Proadrenomedullin to Identify Disease Severity and Treatment Response to Sepsis-a Secondary Analysis of a Large Randomised Controlled Trial. Crit. Care 2018, 22, 79.
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R.; Filippini, C.; Mengozzi, G.; Brazzi, L. Effectiveness of Mid-Regional Pro-Adrenomedullin (MR-ProADM) as Prognostic Marker in COVID-19 Critically Ill Patients: An Observational Prospective Study. PLoS ONE 2021, 16, e0246771.
- Sozio, E.; Tascini, C.; Fabris, M.; D’Aurizio, F.; De Carlo, C.; Graziano, E.; Bassi, F.; Sbrana, F.; Ripoli, A.; Pagotto, A.; et al. MR-ProADM as Prognostic Factor of Outcome in COVID-19 Patients. Sci. Rep. 2021, 11, 5121.
- Yamauchi, A.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Igarashi, K.; Toriyama, Y.; Tanaka, M.; Liu, T.; Xian, X.; et al. Functional Differentiation of RAMP2 and RAMP3 in Their Regulation of the Vascular System. J. Mol. Cell. Cardiol. 2014, 77, 73–85.
- Kitamura, K. Developmental Research of Adrenomedullin. Adrenomedullin No Tenkaikenkyu. Shinzo 2013, 45, 1496–1502. (In Japanese)
- Eto, T.; Kitamura, K. Adrenomedullin and Its Role in Renal Diseases. Nephron 2001, 89, 121–134.
- Ueda, S.; Nishio, K.; Minamino, N.; Kubo, A.; Akai, Y.; Kangawa, K.; Matsuo, H.; Fujimura, Y.; Yoshioka, A.; Masui, K.; et al. Increased Plasma Levels of Adrenomedullin in Patients with Systemic Inflammatory Response Syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 132–136.
- Ashizuka, S.; Kawakami, H.; Kitamura, K. Plasma Adrenomedullin Levels as a Novel Biomarker in Inflammatory Bowel Disease. Enshouseichousikkan Ni Okeru Shinki Biomarker to Shiteno Kecchu Adrenomedullin Noudo No Kentou. J. Jpn. Soc. Gastroenterol. 2019, 116, A688. (In Japanese)
- Minamino, N.; Kikumoto, K.; Isumi, Y. Regulation of Adrenomedullin Expression and Release. Microsc. Res. Tech. 2002, 57, 28–39.
- Kubo, A.; Minamino, N.; Isumi, Y.; Katafuchi, T.; Kangawa, K.; Dohi, K.; Matsuo, H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J. Biol. Chem. 1998, 273, 16730–16738.
- Shindo, T.; Kurihara, H.; Maemura, K.; Kurihara, Y.; Kuwaki, T.; Izumida, T.; Minamino, N.; Ju, K.H.; Morita, H.; Oh-Hashi, Y.; et al. Hypotension and Resistance to Lipopolysaccharide-Induced Shock in Transgenic Mice Overexpressing Adrenomedullin in Their Vasculature. Circulation 2000, 101, 2309–2316.
- Yang, S.; Zhou, M.; Fowler, D.E.; Wang, P. Mechanisms of the Beneficial Effect of Adrenomedullin and Adrenomedullin-Binding protein-1 in Sepsis: Down-Regulation of Proinflammatory Cytokines. Crit. Care Med. 2002, 30, 2729–2735.
- Marutsuka, K.; Hatakeyama, K.; Sato, Y.; Yamashita, A.; Sumiyoshi, A.; Asada, Y. Immunohistological Localization and Possible Functions of Adrenomedullin. Hypertens. Res. 2003, 26, S33–S40.
- Martínez-Herrero, S.; Martínez, A. Adrenomedullin Regulates Intestinal Physiology and Pathophysiology. Domest. Anim. Endocrinol. 2016, 56, S66–S83.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
4 times
(View History)
Update Date:
12 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





