
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hossein Tabatabaeian | + 1250 word(s) | 1250 | 2021-09-09 08:31:35 | | | |
| 2 | Amina Yu | Meta information modification | 1250 | 2021-09-22 11:06:56 | | |
Video Upload Options
Chemoresistance remains a lethal challenge in the realm of cancer biology and clinics. Various determinants with their modes of action have been reported with clinical implications. However, many patients regrettably die due to chemoresistance-induced failure in treatment.
1. Cancer and Chemoresistance
Cancer is one of the major causes of death globally, accounting for 10 million deaths in 2020 [1]. The most common cancers and those responsible for deaths reported in 2020 in both men and women are shown in Figure 1, which highlights a major concern regarding the number of humans affected by cancer worldwide.

Surgery, hormone therapy, gene therapy, immunotherapy, radiation therapy, laser therapy, combination therapy, and targeted therapy are the major cancer treatments available, with chemotherapy being the most common and promising treatment for cancer management [2][3][4]. Despite the progress made in cancer treatment, resistance to chemotherapeutic drugs continues to be a major problem in cancer therapy ( Figure 2 ) and is responsible for most relapses and poor survival outcomes in patients.
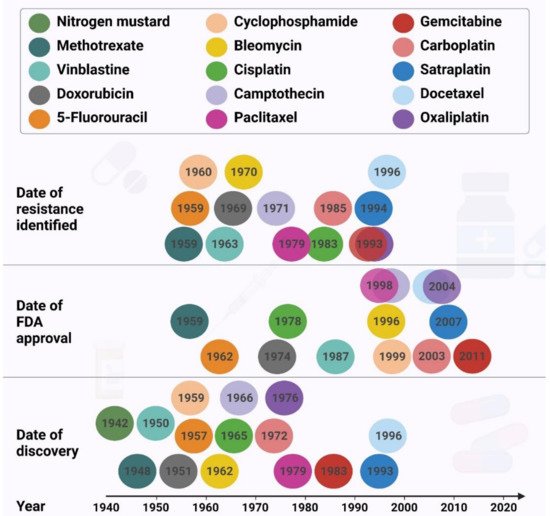
The resistance to the chemotherapeutic regimens has been reported for almost all the drugs used to treat the most lethal cancers. The resistance to doxorubicin [5], paclitaxel [6][7][8], 5-fluorouracil [9], cyclophosphamide [10][11] and carboplatin [12] have been shown to cause cancer recurrence in breast cancer associated with poorer prognosis and shorter survival. Resistance to 5-fluorouracil [13], cisplatin [14][15][16], docetaxel [17][18] and oxaliplatin [19] result in the same outcomes in gastric cancer patients. Moreover, the resistance to 5-fluorouracil [20], irinotecan [21], and oxaliplatin [22] in colorectal cancer, cisplatin [23][24], carboplatin [25][26], paclitaxel 23] and docetaxel [27][28] in lung, and gemcitabine [29], oxaliplatin [30], cisplatin [28][31] and doxorubicin [28] strongly indicate that the chemoresistance phenomenon intensively threatens the health and survival of cancer patients. Approximately 80–90% of mortality in cancer patients is directly or indirectly attributed to drug resistance [32]. Resistance can be restricted to a specific drug, or different drugs with independent modes of action, named multidrug resistance (MDR).
2. Mechanisms of Chemoresistance in Cancer
The effectiveness of chemotherapeutic drugs depends on their successful entry into the cells at an optimum intracellular concentration. Drug influx is affected by the location of the tumor within the body, size of the tumor, physics of the tumor site, the structure and function of the tumor vasculature, necrosis of the tumor, transport properties of the drug as it moves through microvessel walls and in the extravascular tissue, alterations in binding properties and number of drug uptake transport systems (influx pumps), mode of drug diffusion, and absorption of the drug and intracellular pH [32][33].
EMT is the process by which epithelial cells acquire a mesenchymal phenotype. They lose cell polarity and cell-to-cell adhesion and gain migratory and invasive properties, thus promoting metastasis. EMT is related to cancer progression, metastasis and mediates drug resistance to chemotherapy. Several factors like reduced expression of epithelial markers E-cadherin and occludin and increased expression of mesenchymal markers vimentin, fibronectin and N-cadherin, tumor microenvironment, hypoxia and signal transduction pathways (WNT, Notch, Hedgehog) can trigger EMT, leading to drug resistance [34]. EMT-inducing transcriptional factors (EMT-TFs) play a role in drug resistance. Overexpression of EMT-TFs like Twist, Snail, Slug, Zinc finger E-box binding homeobox 1 (ZEB1) and Forkhead box C2 (FOXC2) are known to induce drug resistance in breast cancer [35]. ZEB1 induces EMT by suppressing the epithelial phenotype by repressing epithelial microRNAs such as miRNA-200 family members [36]. Some of the EMT-TFs promote resistance by enhancing drug efflux by ABC transporters. Overexpression of ATP-binding cassette C5 transporter (ABCC5) correlates with Forkhead box M1 (FOXM1) in paclitaxel-resistant nasopharyngeal carcinoma cells. Down-regulation of FOXM1 or ABCC5 reduces drug efflux and leads to cell death by paclitaxel [37].
Anticancer drugs require metabolic activation, and thus cancer cells can develop resistance through drug inactivation or reduced drug activation. Drug activation and inactivation are seen in the glutathione S-transferase (GST) superfamily, a group of detoxifying enzymes that catalyze the conjugation of glutathione (GSH) to electrophilic compounds. GST enzymes induce drug resistance through direct detoxification of cancer drugs or by inhibiting the MAPK pathway [38]. Another mode of drug inactivation occurs through the cytochrome P450 (CYP) system. The CYP system consists of two classes. Class I includes enzymes CYP1A1, CYP1A2, CYP2E1, and CYP3A4, which are involved in the metabolism of drugs and procarcinogens. Class II consists of the enzymes CYP2B6, CYP2C9, CYP2C19, and CYP2D6, which are involved in drug metabolism. It is suggested that mutations in these enzymes would result in the breakdown and secretion of the drugs, thus reducing their optimum concentration leading to drug resistance [39][40]. CYP450 class I metabolizing enzymes are shown to inactivate the drug Irinotecan, a topoisomerase I inhibitor used for colon cancer treatment [41]. The DNA-binding glycopeptide drug bleomycin is inactivated by bleomycin hydrolase. Cancers resistant to bleomycin have high levels of this enzyme, whereas sensitive tumors (germ cell cancers, lymphomas, squamous carcinomas) have low levels [42].
Inducing DNA damage is one of the modes of action of chemotherapeutic drugs to kill cancer cells. If the cells are able to repair the DNA damage caused, they will escape cell death and will develop chemoresistance. DNA damage response (DDR) mechanisms can reverse the drug-induced damage.
3. Strategies to Combat Chemoresistance in Cancer
High-throughput pharmacogenomics and CRISPR screens are being used to investigate populations of cancer cells carrying sensitivity biomarkers and unexpectedly resistant (UNRES) cell lines for unique genetic alterations that may drive resistance. In one study, genomics of drug sensitivity in cancer (GDSC) and clinical trials reporting program (CTRP) datasets were analyzed to find UNRES cases and identify putative resistance biomarkers. Interrogating the foundations of drug resistance with publicly available CRISPR phenotypic assays assists in ranking resistance drivers and offering hypotheses for drug combinations [43]. The EGFR T790M mutation and PTEN loss in lung adenocarcinoma cells treated with EGFR inhibitors have been proposed as the resistance biomarkers based on their hypothesis.
Natural products with their diverse chemical structures and pharmacological benefits can serve as substrates to treat drug resistance. Natural products do so by two approaches. The first is they reduce drug efflux and thus maintain optimum concentration of the drug. The P-gp transporter protein is responsible for efflux of cancer drugs. Binding of P-gp to the drug results in activation of its ATP-binding domain and hydrolysis of ATP causing a change in the shape of P-pg, leading to drug efflux. Natural products that can inhibit the action of the P-gp transport system are being developed. For example, tanshinone microemulsion can significantly reverse drug resistance of K562/ADM cells by inhibiting the P-gp efflux pump effect and increasing the intracellular concentration of chemotherapeutic drugs. Other natural products effective in combating drug resistance include tetrandrine, quercetin, grape-seed polyphenols, and tea polyphenol [44].
The second approach is they induce non-apoptotic cell death in cancer cells. Natural products induce non-apoptotic cell death processes like necroptosis (Shikonin and its analogs, MAM), autophagy (Arsenic trioxide, G. lucidum triterpene, resveratrol, oridonin, allicin), methuosis (chalcone; ginsenoside; curcumin; quercetin) and oncosis (sanguinarine, solamargine, artesunate, rosin) in cancer cells overcoming chemoresistance [45].
Fourteen single compounds shown to be able to overcome cancer cell drug resistance are evodiamine, peiminine, isorhynchophylline, berberine, ephedrine, ginsenoside Rb1, oridonin, oxymatrine, methylether-scutellarein, sodium norcantharidate, phenyl-propanoid glycoside, retinoic acid, schizandrin A, and baicalin [44]. There exists an inverse correlation between consumption of dietary polyphenols and the risk of cancer. Polyphenols possess antioxidant capacity and inhibit activation of procarcinogens, cancer cell proliferation, metastasis, angiogenesis and drug efflux transporters. They also induce apoptosis in cancer cells and modulate immune responses and inflammatory cascades [46].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249.
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med Sci. 2012, 9, 193.
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160.
- Smith, L.; Watson, M.B.; O’Kane, S.L.; Drew, P.J.; Lind, M.J.; Cawkwell, L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer Ther. 2006, 5, 2115–2120.
- Harris, L.N.; Broadwater, G.; Lin, N.U.; Miron, A.; Schnitt, S.J.; Cowan, D.; Lara, J.; Bleiweiss, I.; Berry, D.; Ellis, M. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: Results from CALGB 9342. Breast Cancer Res. 2006, 8, 1–12.
- Murray, S.; Briasoulis, E.; Linardou, H.; Bafaloukos, D.; Papadimitriou, C. Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012, 38, 890–903.
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234.
- Vulsteke, C.; Pfeil, A.M.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Peeters, M.; van Dam, P.; Dieudonné, A.-S.; Hatse, S. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res. Treat. 2014, 147, 557–570.
- Porkka, K.; Blomqvist, C.; Rissanen, P.; Elomaa, I.; Pyrhönen, S. Salvage therapies in women who fail to respond to first-line treatment with fluorouracil, epirubicin, and cyclophosphamide for advanced breast cancer. J. Clin. Oncol. 1994, 12, 1639–1647.
- Sládek, N.E.; Kollander, R.; Sreerama, L.; Kiang, D.T. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: A retrospective study. Cancer Chemother. Pharmacol. 2002, 49, 309–321.
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014, 5, e1257.
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569.
- Huang, D.; Duan, H.; Huang, H.; Tong, X.; Han, Y.; Ru, G.; Qu, L.; Shou, C.; Zhao, Z. Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci. Rep. 2016, 6, 1–12.
- Wang, X.; Xu, Z.; Sun, J.; Lv, H.; Wang, Y.; Ni, Y.; Chen, S.; Hu, C.; Wang, L.; Chen, W. Cisplatin resistance in gastric cancer cells is involved with GPR30-mediated epithelial-mesenchymal transition. J. Cell. Mol. Med. 2020, 24, 3625–3633.
- Mora-Lagos, B.; Cartas-Espinel, I.; Riquelme, I.; Parker, A.C.; Piccolo, S.R.; Viscarra, T.; Reyes, M.E.; Zanella, L.; Buchegger, K.; Ili, C. Functional and transcriptomic characterization of cisplatin-resistant AGS and MKN-28 gastric cancer cell lines. PLoS ONE 2020, 15, e0228331.
- Urano, N.; Fujiwara, Y.; Doki, Y.; Kim, S.; Miyoshi, Y.; Noguchi, S.; Miyata, H.; Takiguchi, S.; Yasuda, T.; Yano, M. Clinical significance of class III β-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int. J. Oncol. 2006, 28, 375–381.
- Li, X.; Yao, R.; Yue, L.; Qiu, W.; Qi, W.; Liu, S.; Yao, Y.; Liang, J. FOXM 1 mediates resistance to docetaxel in gastric cancer via up-regulating Stathmin. J. Cell. Mol. Med. 2014, 18, 811–823.
- Hu, Y.; Su, Y.; Lei, X.; Zhao, H.; Wang, L.; Xu, T.; Guo, J.; Yang, W.; Zhang, X. LINC00641/miR-582-5p mediate oxaliplatin resistance by activating autophagy in gastric adenocarcinoma. Sci. Rep. 2020, 10, 1–11.
- Wang, B.; Ma, N.; Zheng, X.; Li, X.; Ma, X.; Hu, J.; Cao, B. GDF15 Repression Contributes to 5-Fluorouracil Resistance in Human Colon Cancer by Regulating Epithelial-Mesenchymal Transition and Apoptosis. BioMed Res. Int. 2020, 2020, 2826010.
- Nielsen, D.L.; Palshof, J.A.; Brünner, N.; Stenvang, J.; Viuff, B.M. Implications of ABCG2 expression on irinotecan treatment of colorectal cancer patients: A review. Int. J. Mol. Sci. 2017, 18, 1926.
- Lin, Q.; Luo, L.; Wang, H. A new oxaliplatin resistance-related gene signature with strong predicting ability in colon cancer identified by comprehensive profiling. Front. Oncol. 2021, 11, 644956.
- Huang, W.-C.; Kuo, K.-T.; Wang, C.-H.; Yeh, C.-T.; Wang, Y. Cisplatin resistant lung cancer cells promoted M2 polarization of tumor-associated macrophages via the Src/CD155/MIF functional pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–17.
- Ballestreri, É.; Simon, D.; de Souza, A.P.; Grott, C.S.; Nabinger, D.D.; Dihl, R.R.; Grivicich, I. Resistance mechanism to cisplatin in NCI-H460 non-small cell lung cancer cell line: Investigating apoptosis, autophagy, and cytogenetic damage. Cancer Drug Resist. 2018, 1, 72–81.
- Liu, X.; Pan, C.-G.; Luo, Z.-Q. High expression of NFAT2 contributes to carboplatin resistance in lung cancer. Exp. Mol. Pathol. 2019, 110, 104290.
- Cosaert, J.; Quoix, E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer 2002, 87, 825–833.
- Zaman, G.J.; Versantvoort, C.H.; Smit, J.J.; Eijdems, E.W.; de Haas, M.; Smith, A.J.; Broxterman, H.J.; Mulder, N.H.; de Vries, E.G.; Baas, F. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993, 53, 1747–1750.
- Sosa Iglesias, V.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug resistance in non-small cell lung cancer: A potential for NOTCH targeting? Front. Oncol. 2018, 8, 267.
- Wang, N.; Wang, S.; Li, M.-Y.; Hu, B.-G.; Liu, L.-P.; Yang, S.-L.; Yang, S.; Gong, Z.; Lai, P.B.; Chen, G.G. Cancer stem cells in hepatocellular carcinoma: An overview and promising therapeutic strategies. Ther. Adv. Med. Oncol. 2018, 10, 1758835918816287.
- Zhang, X.; Xu, P.; Ni, W.; Fan, H.; Xu, J.; Chen, Y.; Huang, W.; Lu, S.; Liang, L.; Liu, J. Downregulated DYRK2 expression is associated with poor prognosis and Oxaliplatin resistance in hepatocellular carcinoma. Pathol.-Res. Pract. 2016, 212, 162–170.
- Ru, Y.; Chen, X.-J.; Guo, W.-Z.; Gao, S.-G.; Qi, Y.-J.; Chen, P.; Feng, X.-S.; Zhang, S.-J. Neat1_2–sFPQ axis mediates cisplatin resistance in liver cancer cells in vitro. Onco Targets Ther. 2018, 11, 5695.
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339.
- Redmond, K.M.; Wilson, T.R.; Johnston, P.G.; Longley, D.B. Resistance mechanisms to cancer chemotherapy. Front. Biosci. 2008, 13, 5138–5154.
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297.
- Huang, J.; Li, H.; Ren, G. Epithelial-mesenchymal transition and drug resistance in breast cancer. Int. J. Oncol. 2015, 47, 840–848.
- Song, K.-A.; Faber, A.C. Epithelial-to-mesenchymal transition and drug resistance: Transitioning away from death. J. Thorac. Dis. 2019, 11, E82.
- Jiang, Z.-S.; Sun, Y.-Z.; Wang, S.-M.; Ruan, J.-S. Epithelial-mesenchymal transition: Potential regulator of ABC transporters in tumor progression. J. Cancer 2017, 8, 2319.
- Townsend, D.M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375.
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792.
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of drug resistant cancer cells: A matter of combination therapy. Cancers 2018, 10, 483.
- Xu, Y.; Villalona-Calero, M. Irinotecan: Mechanisms of tumor resistance and novel strategies for modulating its activity. Ann. Oncol. 2002, 13, 1841–1851.
- Chen, J.; Chen, Y.; He, Q. Action of bleomycin is affected by bleomycin hydrolase but not by caveolin-1. Int. J. Oncol. 2012, 41, 2245–2252.
- Ayestaran, I.; Galhoz, A.; Spiegel, E.; Sidders, B.; Dry, J.R.; Dondelinger, F.; Bender, A.; McDermott, U.; Iorio, F.; Menden, M.P. Identification of Intrinsic Drug Resistance and Its Biomarkers in High-Throughput Pharmacogenomic and CRISPR Screens. Patterns 2020, 1, 100065.
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome cancer cell drug resistance using natural products. Evid.-Based Complementary Altern. Med. 2015, 2015, 767136.
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural products to prevent drug resistance in cancer chemotherapy: A review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27.
- Hussain, S.A.; Sulaiman, A.A.; Balch, C.; Chauhan, H.; Alhadidi, Q.M.; Tiwari, A.K. Natural polyphenols in cancer chemoresistance. Nutr. Cancer 2016, 68, 879–891.




