
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lingchao Xiang | + 1606 word(s) | 1606 | 2021-08-09 14:48:32 | | | |
| 2 | Vicky Zhou | Meta information modification | 1606 | 2021-09-22 08:31:10 | | | | |
| 3 | Rita Xu | Meta information modification | 1606 | 2024-05-23 07:23:34 | | |
Video Upload Options
Magnetic material nanoparticles (MNPs) have been widely used in the detection and treatment of bacterial infections as detection agents and therapeutics. Infections caused by pathogenic bacteria, especially multidrug-resistant bacteria, have become a serious worldwide public health problem. Early diagnosis and treatment can effectively prevent the adverse effects of such infections. Therefore, there is an urgent need to develop effective methods for the early detection, prevention, and treatment of diseases that are caused by bacterial infections.
1. Introduction
Bacterial infections, especially those caused by multidrug-resistant bacteria, have become a global public health concern [1]. The emergence of antimicrobial resistance is largely attributed to the indiscriminate and often abusive use of antimicrobials [2]. It was clinically found that due to the abuse of antibiotics, there is a lack of effective antibacterial drugs to treat bacterial infections [3]. It is estimated that deaths due to infectious diseases that are associated with resistant pathogens will lead to 10 million deaths per year by 2050 [4]. Therefore, there is an urgent need to develop non-antibiotic drugs to treat multidrug-resistant bacterial infections [5].
The early detection and identification of bacterial infections are also major clinical challenges [6]. Moreover, the traditional detection and identification methods are time-consuming and tedious. In order to overcome the shortcomings of traditional antibacterial treatment and detection methods, various metals and metal oxide nanoparticles have been used for bacterial detection and treatment [7]. MNPs have been widely used in the biological field in recent years due to their physical properties, good biocompatibility, and high binding capacity [8][9][10], including in vivo and in vitro bacterial detection and separation imaging [11], as well as the treatment of pathogenic bacteria. For example, magnetic materials were used to synthesize new structures [12] and new magnetic material composite nanoparticles with improved structural stability [13], biological activity [14], and antibacterial properties [15][16][17][18] to realize the separability and recyclability of MNPs [19][20][21].
2. MNPs as Antibacterial Therapies
The discovery of penicillin in 1928 by Alexander Fleming [22] ushered in the “antibiotic era”; penicillin was truly a miracle drug: uniformly fatal infections could be cured. By the mid-1940s after the antibacterial efficacy of penicillin was clinically proven, antibiotics have begun to play a role in various diseases. The discovery of antibiotics has brought effective cures for many diseases to mankind [23]. Yet, as human beings progress, bacteria are constantly evolving. When antibiotics are widely used, they are also constantly losing their antibacterial efficacy, leading to the problem of antibiotic resistance [24][25]. Therefore, the development of new antibiotic therapies is one of the important strategies that are used to combat super-resistant bacteria. In particular, finding new sterilization mechanisms has become a new hot spot in the development of new therapies for the treatment of infections.
Along with the extensive range of exotic NPs applications, the investigation of MNPs in vitro has ushered modern antibacterial studies into an increasingly attractive research area. The great potential of engineered MNPs in the treatment of various resistant bacteria has reduced the threat of deadly bacterial infections [26]. The properties of MNPs allow them to be guided around the body by a magnetic field or into magnetic implants. This opens up the potential to combine various biological materials with nanoparticles, which can then be directed into the body for treatment [27].
2.1. Magnetic Hyperthermia
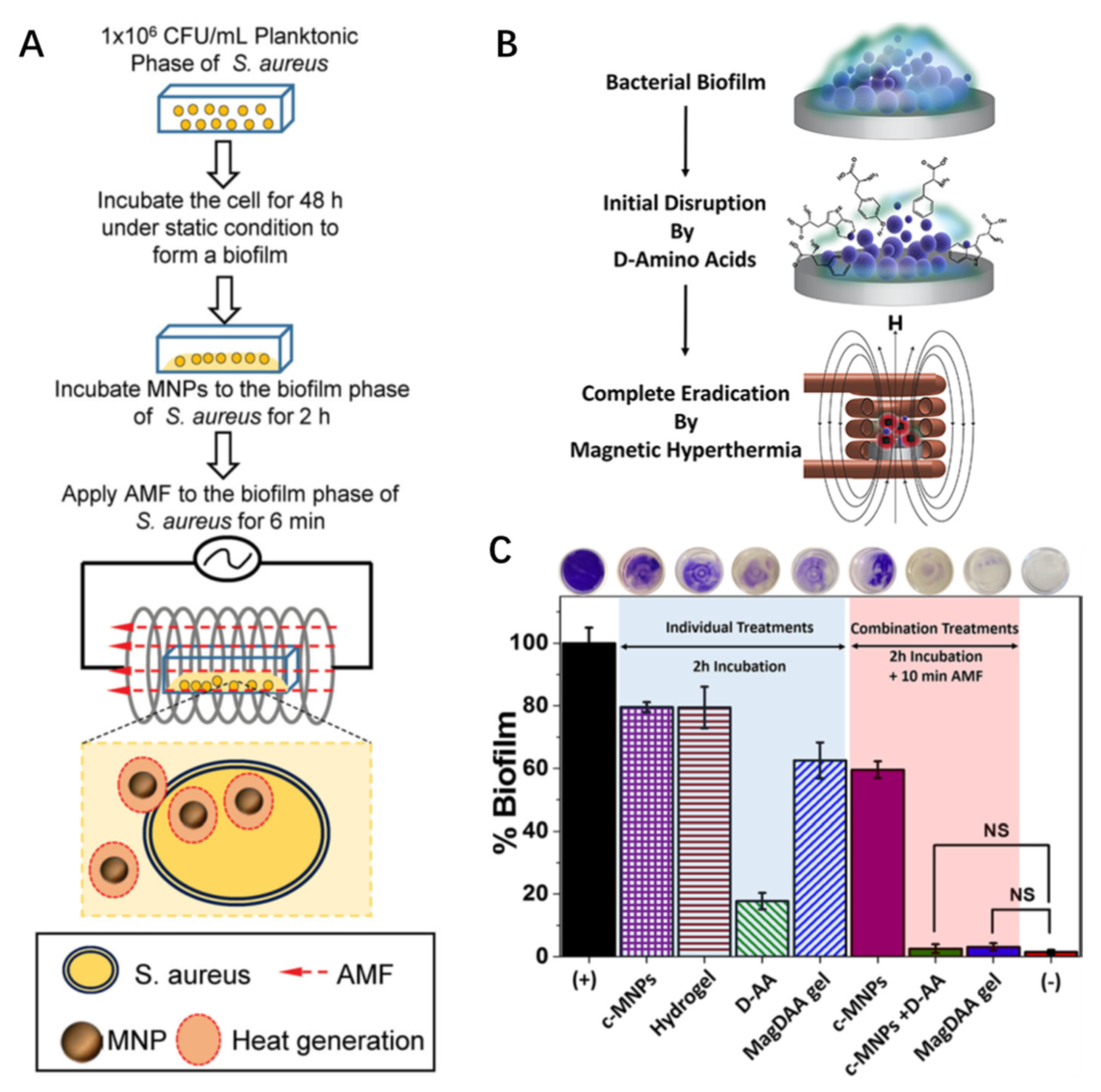
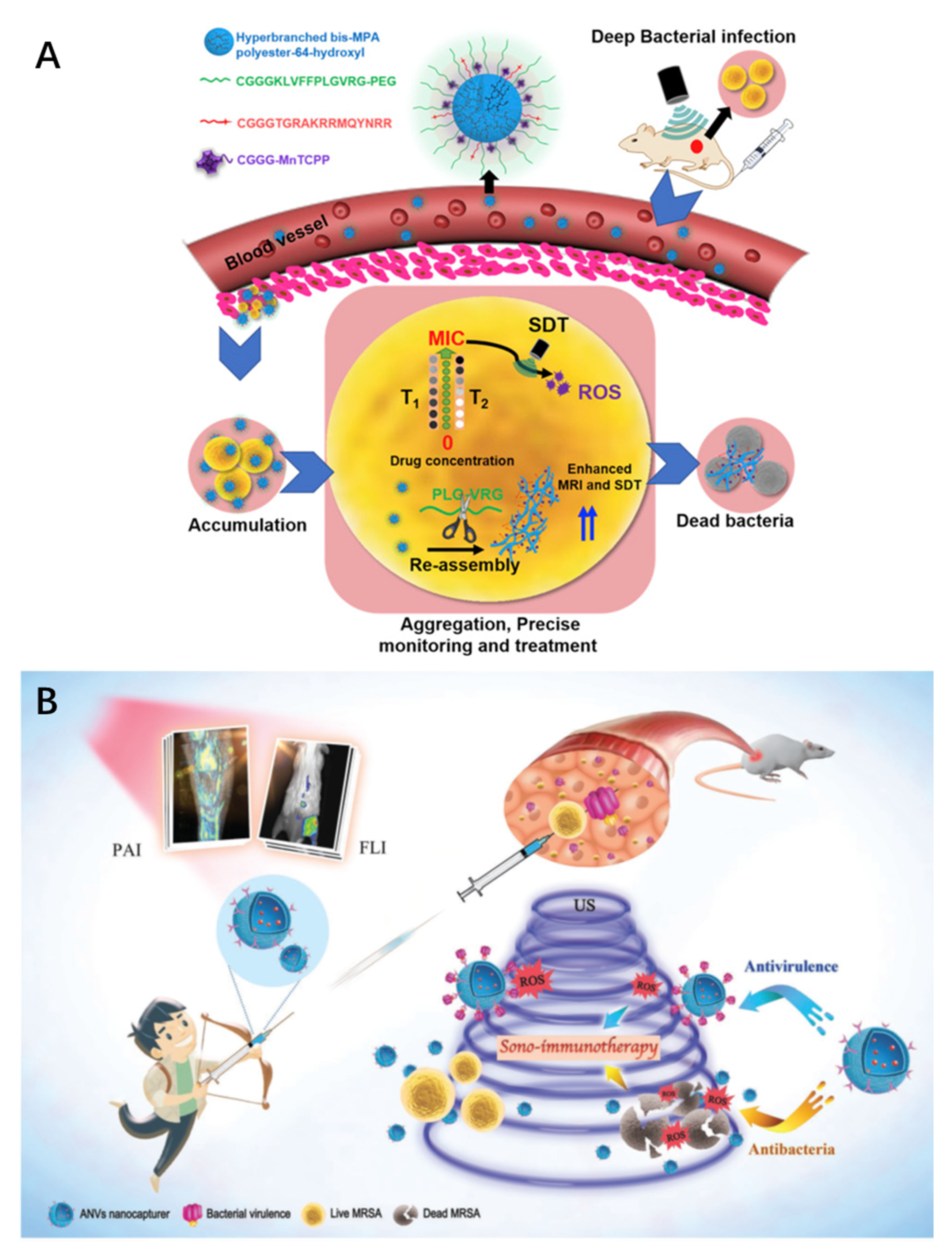
2.2. Multifunctional MNPs for Theranostics
3. Summary and Perspectives
References
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738.
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839.
- Olesen, S.W.; Barnett, M.L.; MacFadden, D.R.; Brownstein, J.S.; Hernandez-Diaz, S.; Lipsitch, M.; Grad, Y.H. The distribution of antibiotic use and its association with antibiotic resistance. Elife 2018, 7, e39435.
- Campanini-Salinas, J.; Andrades-Lagos, J.; Melia-Raipan, J.; Vasquez-Velasquez, D. Novel Classes of Antibacterial Drugs in Clinical Development, a Hope in a Post-antibiotic Era. Curr. Top. Med. Chem. 2018, 18, 1188–1202.
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.E.; Nicolau, D.P.; Postma, M.J.; et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017, 43, 1187–1197.
- Bhatia, B.D.; Basu, S. Newer diagnostic tests for bacterial diseases. Indian J. Pediatr. 2007, 74, 673–677.
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Healthc. Mater. 2018, 7, e1701392.
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.Z.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014.
- Xu, C.; Akakuru, O.U.; Zheng, J.J.; Wu, A.G. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 00141.
- Ma, Y.; Chen, T.; Iqbal, M.Z.; Yang, F.; Hampp, N.; Wu, A.; Luo, L. Applications of magnetic materials separation in biological nanomedicine. Electrophoresis 2019, 40, 2011–2028.
- Bell, C.S.; Mejias, R.; Miller, S.E.; Greer, J.M.; McClain, M.S.; Cover, T.L.; Giorgio, T.D. Magnetic Extraction of Acinetobacter baumannii Using ColistinFunctionalized gamma-Fe2O3/Au Core/Shell Composite Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 26719–26730.
- Bruschi, M.L.; Sica de Toledo, L.d.A. Pharmaceutical Applications of Iron-Oxide Magnetic Nanoparticles. Magnetochemistry 2019, 5, 50.
- Matai, I.; Garg, M.; Rana, K.; Singh, S. Polydopamine functionalized hydrogel beads as magnetically separable antibacterial materials. RSC Adv. 2019, 9, 13444–13457.
- Chandrappa, M.; Swathi, K.; Vijayalakshmi, U.; Pullela, P.K.; Kumar, S.G. Magnetic Nanoparticle Assisted Bulk Scale Synthesis of Quinazoline Synthon. Adv. Sci. Lett. 2018, 24, 5936–5941.
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptula, P.; Wilczewska, A.Z.; Misiak, P.; Durnas, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP2-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81.
- Abdulhady, Y.A.M.; El-Shazly, M.M.; El-Kased, R.F. Evaluation of antibacterial activity and toxic metal removal of chemically synthesized magnetic iron oxide titanium coated nanoparticles and application in bacterial treatment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 205–212.
- Chen, C.; Xia, D.-L.; Guo, L.-Y.; Chen, Y.-P.; Li, X.-D.; Wang, Y.-F.; Zhang, D.; Wang, Y.-Y.; Zhang, Y.-X.; He, H.; et al. Extracorporeal magnetic approach to reduce the unwanted side-effects and improve antibacterial activity of Ag/Fe3O4 nanocomposites in rat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2029–2036.
- Ai, C.; Wu, S.; Li, L.; Lei, Y.; Shao, X. Novel magnetically separable gamma-Fe2O3/Ag/AgCl/g-C3N4 composite for enhanced disinfection under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123981.
- Lei, J.; Chen, S.; Yanyi, W.; Zhongjie, Z.; Jia, L.; Sirui, H.; Shu, H.; Xiufeng, L.; Wei, S. Antibacterial activity and long-term stable antibacterial performance of nisin grafted magnetic GO nanohybrids. Mater. Sci. Eng. C 2020, 111, 110809.
- Akter, N.; Chowdhury, L.; Uddin, J.; Ullah, A.K.M.A.; Shariare, M.H.; Azam, M.S. N-halamine functionalization of polydopamine coated Fe3O4 nanoparticles for recyclable and magnetically separable antimicrobial materials. Mater. Res. Express 2018, 5, 115007.
- Al-Jabari, M.H.; Sulaiman, S.; Ali, S.; Barakat, R.; Mubarak, A.; Khan, S.A. Adsorption study of levofloxacin on reusable magnetic nanoparticles: Kinetics and antibacterial activity. J. Mol. Liq. 2019, 291, 111249.
- Diggins, F.W.E. The true history of the discovery of penicillin, with refutation of the misinformation in the literature. Br. J. Biomed. Sci. 1999, 56, 83–93.
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641.
- Li, J.; Sexton, P.M. Targeting Antibiotic Resistance: From Diagnostics to Novel Antibiotics. ACS Pharmacol. Transl. Sci. 2020, 3, 371–372.
- Blaskovich, M.A.T. Antibiotics Special Issue: Challenges and Opportunities in Antibiotic Discovery and Development. ACS Infect. Dis. 2020, 6, 1286–1288.
- Allafchian, A.; Hosseini, S.S. Antibacterial magnetic nanoparticles for therapeutics: A review. IET Nanobiotechnol. 2019, 13, 786–799.
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188.
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperth. 2020, 37, 66–75.
- Abenojar, E.C.; Wickramasinghe, S.; Ju, M.; Uppaluri, S.; Klika, A.; George, J.; Barsoum, W.; Frangiamore, S.J.; Higuera-Rueda, C.A.; Samia, A.C.S. Magnetic Glycol Chitin-Based Hydrogel Nanocomposite for Combined Thermal and D-Amino-Acid-Assisted Biofilrn Disruption. ACS Infect. Dis. 2018, 4, 1246–1256.
- Wang, D.; Cheng, D.B.; Ji, L.; Niu, L.J.; Zhang, X.H.; Cong, Y.; Cao, R.H.; Zhou, L.; Bai, F.; Qiao, Z.Y.; et al. Precise magnetic resonance imaging-guided sonodynamic therapy for drug-resistant bacterial deep infection. Biomaterials 2021, 264, 120386.
- Pang, X.; Liu, X.; Cheng, Y.; Zhang, C.; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X.; Liu, G. Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections. Adv. Mater. 2019, 31, e1902530.




